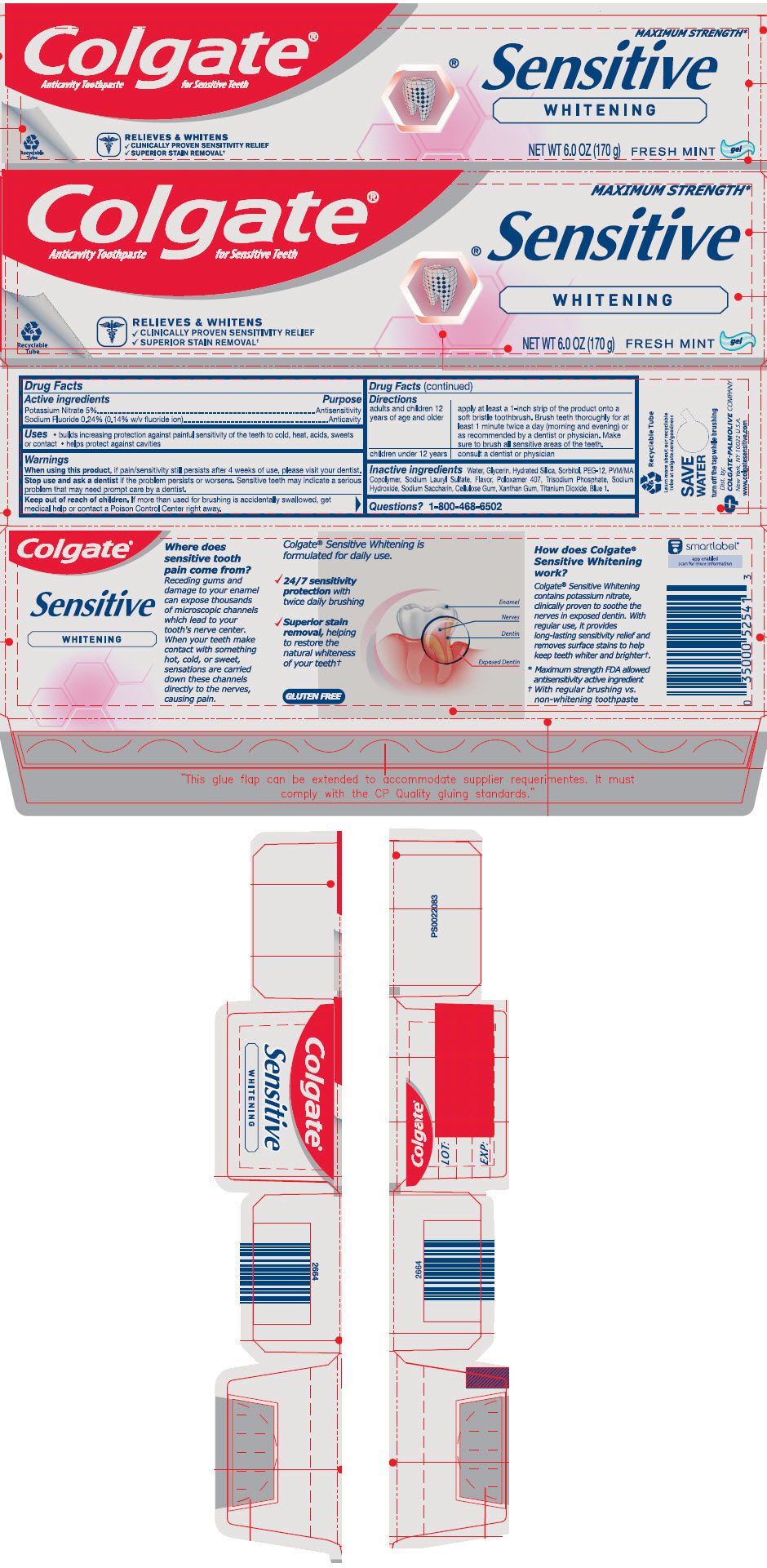
| Active ingredients | Purpose |
| Potassium Nitrate 5% | Antisensitivity |
| Sodium Fluoride 0.24% (0.14% w/v fluoride ion) | Anticavity |
Uses
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
- helps protect against cavities
Warnings
When using this product, if pain/sensitivity still persists after 4 weeks of use, please visit your dentist.
Stop use and ask a dentist if the problem persists or worsens. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist.
Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years of age and older | apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth. |
| children under 12 years | consult a dentist or physician |
Inactive ingredients
Water, Glycerin, Hydrated Silica, Sorbitol, PEG-12, PVM/MA Copolymer, Sodium Lauryl Sulfate, Flavor, Poloxamer 407, Trisodium Phosphate, Sodium Hydroxide, Sodium Saccharin, Cellulose Gum, Xanthan Gum, Titanium Dioxide, Blue 1.
Questions?
1-800-468-6502
Dist. by:
COLGATE-PALMOLIVE COMPANY
New York, NY 10022 U.S.A.
PRINCIPAL DISPLAY PANEL - 170 g Tube Carton
Colgate®
Anticavity Toothpaste for Sensitive Teeth
MAXIMUM STRENGTH*
Sensitive
WHITENING
Recyclable
Tube
RELIEVES & WHITENS
- ✓
- CLINICALLY PROVEN SENSITIVITY RELIEF
- ✓
- SUPERIOR STAIN REMOVAL†
NET WT 6.0 OZ (170 g)
FRESH MINT
gel