Label: INFLAMMATION REDUCTION PACK kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 69176-010-01 - Packager: TMIG, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 18, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Kit Description
-
Ranitidine hydrochloride
Ranitidine hydrochloride (HCl), is a histamine H2-receptor antagonist. Chemically it is N-[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N’-methyl-2-nitro-1,1-ethenediamine, HCl.
It has the following structure:
ranitidine hydrochloride chemical structure
The empirical formula is C13H22N4O3S • HCl, representing a molecular weight of 350.87.Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfur like odor.
Each tablet, for oral administration contains 168 mg or 336 mg of ranitidine hydrochloride equivalent to 150 mg and 300 mg of ranitidine, respectively. Inactive ingredients: D & C Red #30 Aluminum Lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, triethyl citrate, sodium starch glycolate, titanium dioxide and flavoring. The 300 mg also contains: D & C Yellow #10 Aluminum Lake.
Each capsule, for oral administration contains 168 mg or 336 mg of ranitidine hydrochloride equivalent to 150 mg and 300 mg of ranitidine, respectively. Inactive ingredients: Ammonium hydroxide, colloidal silicon dioxide, corn starch, FD & C Blue #1, FD & C Red #40, FD & C Yellow #6, gelatin, magnesium stearate, pharmaceutical glaze, propylene glycol, silicon dioxide, simethicone, sodium lauryl sulfate, sodium starch glycolate, and titanium dioxide.
-
Diclofenac sodium
Diclofenac sodium delayed-release tablets, USP are a benzeneacetic acid derivative. The chemical name is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt. The molecular weight is 318.13. Its molecular formula is C14H10Cl2NNaO2, and it has the following structural formula
78e77986-figure-01
Each enteric-coated tablet for oral administration contains 50 mg or 75 mg of diclofenac sodium, USP. In addition, each tablet contains the following inactive ingredients: aluminum hydrate, colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl acetate phthalate, propylene glycol, silica, sodium alginate, sodium starch glycolate (Type A), stearic acid, synthetic black iron oxide, talc, and titanium dioxide. -
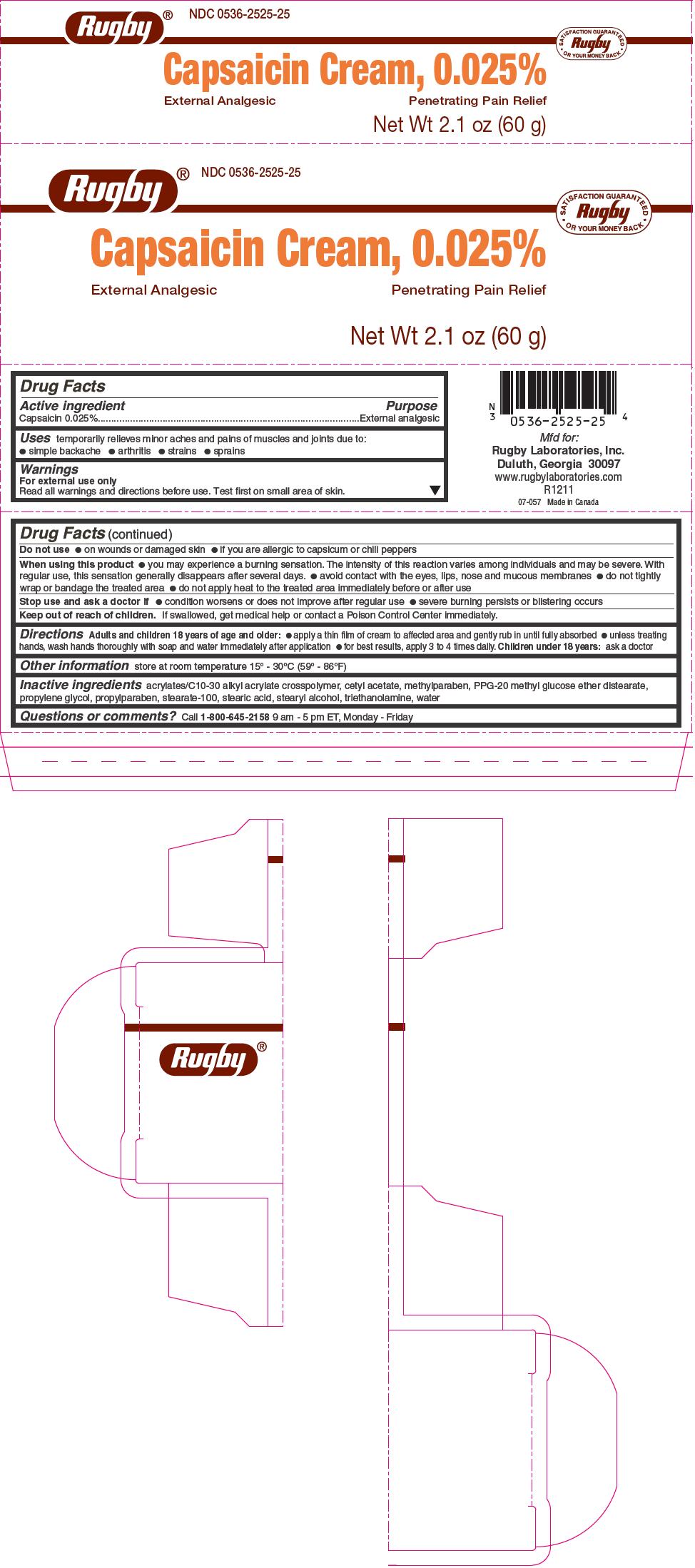
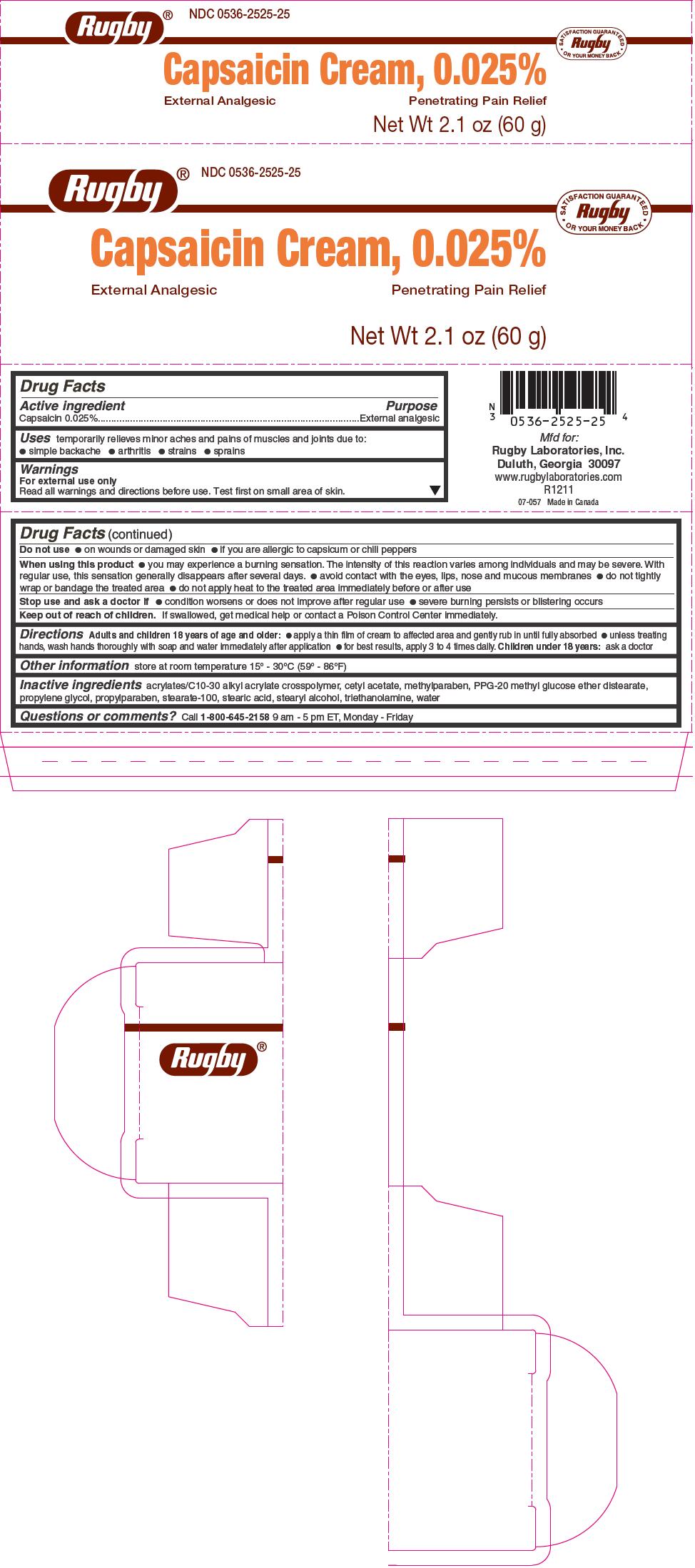
Capsaisin 0.025%
For external use only
Read all warnings and directions before use. Test first on small area of skin.
Do not use
on wounds or damaged skin
if you are allergic to capsicum or chili peppers
When using this product
you may experience a burning sensation. The intensity of this reaction varies among individuals and may be severe. With regular use, this sensation generally disappears after several days.
avoid contact with the eyes, lips, nose and mucous membranes
do not tightly wrap or bandage the treated area
do not apply heat to the treated area immediately before or after use
Stop use and ask a doctor if
condition worsens or does not improve after regular use
severe burning persists or blistering occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately. -
Diclofenac Sodium
Cardiovascular Effects
Cardiovascular Thrombotic EventsClinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see WARNINGS, GI EFFECTS).
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including diclofenac sodium delayed-release tablets, USP should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.Congestive Heart Failure and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs. Diclofenac sodium delayed-release should be used with caution in patients with fluid retention or heart failure.Gastrointestinal (GI) Effects - Risk Of GI Ulceration, Bleeding, And Perforation
NSAIDs, including diclofenac sodium delayed-release, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2%-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects
Caution should be used when initiating treatment with diclofenac sodium delayed-release in patients with considerable dehydration.Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of diclofenac sodium delayed-release in patients with advanced renal disease. Therefore, treatment with diclofenac sodium delayed-release is not recommended in these patients with advanced renal disease. If diclofenac sodium delayed-release therapy must be initiated, close monitoring of the patient's renal function is advisable.
Hepatic Effects
Elevations of one or more liver tests may occur during therapy with diclofenac sodium delayed-release. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continued therapy. Borderline elevations (i.e., less than 3 times the ULN [ULN = the upper limit of the normal range]) or greater elevations of transaminases occurred in about 15% of diclofenac-treated patients. Of the markers of hepatic function, ALT (SGPT) is recommended for the monitoring of liver injury.In clinical trials, meaningful elevations (i.e., more than 3 times the ULN) of AST (GOT) (ALT was not measured in all studies) occurred in about 2% of approximately 5,700 patients at some time during diclofenac treatment. In a large, open-label, controlled trial of 3,700 patients treated for 2-6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of patients and included marked elevations (i.e., more than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3-8 times the ULN), and marked (>8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis.
Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations.
In postmarketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac.
If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), diclofenac sodium delayed-release should be discontinued immediately.
To minimize the possibility that hepatic injury will become severe between transaminase measurements, physicians should inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms), and the appropriate action patients should take if these signs and symptoms appear.
To minimize the potential risk for an adverse liver related event in patients treated with diclofenac sodium delayed-release, the lowest effective dose should be used for the shortest duration possible. Caution should be exercised in prescribing diclofenac sodium delayed-release with concomitant drugs that are known to be potentially hepatotoxic (e.g., antibiotics, anti-epileptics).
Anaphylactic Reactions
As with other NSAIDs, anaphylactic reactions may occur both in patients with the aspirin triad and in patients without known sensitivity to NSAIDs or known prior exposure to diclofenac sodium delayed-release. Diclofenac sodium delayed-release should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs. (See CONTRAINDICATIONS and PRECAUTIONS, PREEXISTING ASTHMA.) Anaphylaxis-type reactions have been reported with NSAID products, including with diclofenac products, such as diclofenac sodium delayed-release. Emergency help should be sought in cases where an anaphylactic reaction occurs.Skin Reactions
NSAIDs, including diclofenac sodium delayed-release, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.Pregnancy
In late pregnancy, as with other NSAIDs, diclofenac sodium delayed-release should be avoided because it may cause premature closure of the ductus arteriosus. - Ranitidine Hydochloride
- Capsaisain cream
- Ranitdine
- Diclofenac
-
Ranitdine
Ranitidine is a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine does not lower serum Ca++ in hypercalcemic states. Ranitidine is not a anticholinergic agent.
Pharmacokinetics
AbsorptionRanitidine tablets and ranitidine capsules are 50% absorbed after oral administration, compared to an intravenous (IV) injection with mean peak levels of 440 to 545 ng/mL occurring 2 to 3 hours after a 150 mg dose. Absorption is not significantly impaired by the administration of food or antacids. Propantheline slightly delays and increases peak blood levels of ranitidine, probably by delaying gastric emptying and transit time. In one study, simultaneous administration of high-potency antacid (150 mmol) in fasting subjects has been reported to decrease the absorption of ranitidine.
Distribution
The volume of distribution is about 1.4 L/kg. Serum protein binding averages 15%.
Metabolism
In humans, the N-oxide is the principal metabolite in the urine; however, this amounts to <4% of the dose. Other metabolites are the S-oxide (1%) and the desmethyl ranitidine (1%). The remainder of the administered dose is found in the stool. Studies in patients with hepatic dysfunction (compensated cirrhosis) indicate that there are minor, but clinically insignificant, alterations in ranitidine half-life, distribution, clearance, and bioavailability.
Excretion
The principal route of excretion is the urine, with approximately 30% of the orally administered dose collected in the urine as unchanged drug in 24 hours. Renal clearance is about 410 mL/min, indicating active tubular excretion. The elimination half-life is 2.5 to 3 hours. Four patients with clinically significant renal function impairment (creatinine clearance 25 to 35 mL/min) administered 50 mg of ranitidine intravenously had an average plasma half-life of 4.8 hours, a ranitidine clearance of 29 mL/min, and a volume of distribution of 1.76 L/kg. In general, these parameters appear to be altered in proportion to creatinine clearance (see DOSAGE AND ADMINISTRATION).
Geriatrics
The plasma half-life is prolonged and total clearance is reduced in the elderly population due to a decrease in renal function. The elimination half-life is 3 to 4 hours. Peak levels average 526 ng/mL following a 150 mg twice daily dose and occur in about 3 hours (see PRECAUTIONS: GERIATRIC USE and DOSAGE AND ADMINISTRATION: DOSAGE ADJUSTMENT FOR PATIENTS WITH IMPAIRED RENAL FUNCTION).
Pediatrics
There are no significant differences in the pharmacokinetic parameter values for ranitidine in pediatric patients (from 1 month up to 16 years of age) and healthy adults when correction is made for body weight. The average bioavailability of ranitidine given orally to pediatric patients is 48% which is comparable to the bioavailability of ranitidine in the adult population. All other pharmacokinetic parameter values (t1/2, Vd, and CL) are similar to those observed with intravenous ranitidine use in pediatric patients. Estimates of Cmax and Tmax are displayed in Table 1.
-
Ranitdine
Ranitidine is indicated in:
Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year.
The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with ranitidine 150 mg two times a day.
Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with ranitidine 150 mg 4 times daily.
Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis. - Ranitidine
-
Ranitdine
Active Duodenal Ulcer
The current recommended adult oral dosage of ranitidine for duodenal ulcer is 150 mg twice daily. An alternative dosage of 300 mg once daily after the evening meal or at bedtime can be used for patients in whom dosing convenience is important. The advantages of one treatment regimen compared to the other in a particular patient population have yet to be demonstrated (see CLINICAL PHARMACOLOGY: CLINICAL TRIALS: ACTIVE DUODENAL ULCER). Smaller doses have been shown to be equally effective in inhibiting gastric acid secretion in US studies, and several foreign trials have shown that 100 mg twice daily is as effective as the 150 mg dose.Antacid should be given as needed for relief of pain (see CLINICAL PHARMACOLOGY: PHARMACOKINETICS).
-
INGREDIENTS AND APPEARANCE
INFLAMMATION REDUCTION PACK
inflammation reduction pack kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69176-010 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69176-010-01 1 in 1 KIT; Type 0: Not a Combination Product 08/18/2015 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 Part 2 1 Part 3 1 Part 1 of 3 RUGBY CAPSAICIN EXTERNAL ANALGESIC
capsaicin creamProduct Information Item Code (Source) NDC:0536-2525 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength PPG-20 METHYL GLUCOSE ETHER DISTEARATE (UNII: 0057334FAB) CETYL ACETATE (UNII: 4Q43814HXS) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARETH-100 (UNII: 4OH5W9UM87) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/01/2012 Part 2 of 3 DICLOFENAC SODIUM
diclofenac sodium tablet, delayed releaseProduct Information Item Code (Source) NDC:0228-2551 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICLOFENAC SODIUM (UNII: QTG126297Q) (DICLOFENAC - UNII:144O8QL0L1) DICLOFENAC SODIUM 75 mg Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM ALGINATE (UNII: C269C4G2ZQ) STEARIC ACID (UNII: 4ELV7Z65AP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code R;551 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074514 03/26/1996 Part 3 of 3 RANITIDINE HYDROCHLORIDE
ranitidine hydrochloride tablet, film coatedProduct Information Item Code (Source) NDC:0781-1883 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength PEPPERMINT (UNII: V95R5KMY2B) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) D&C RED NO. 30 (UNII: 2S42T2808B) HYDROXYPROPYL CELLULOSE (TYPE H) (UNII: RFW2ET671P) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score score with uneven pieces Shape ROUND Size 8mm Flavor Imprint Code GG;705 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074655 10/22/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074514 08/18/2015 Labeler - TMIG, Inc. (036572986) Registrant - TMIG, Inc. (036572986) Establishment Name Address ID/FEI Business Operations EPM Packaging 079124340 repack(69176-010) Establishment Name Address ID/FEI Business Operations Garcoa, Inc. 103039178 manufacture(0536-2525) Establishment Name Address ID/FEI Business Operations Sandoz Inc. 110342024 manufacture(0781-1883) Establishment Name Address ID/FEI Business Operations Actavis Elizabeth LLC 623114928 manufacture(0228-2551)