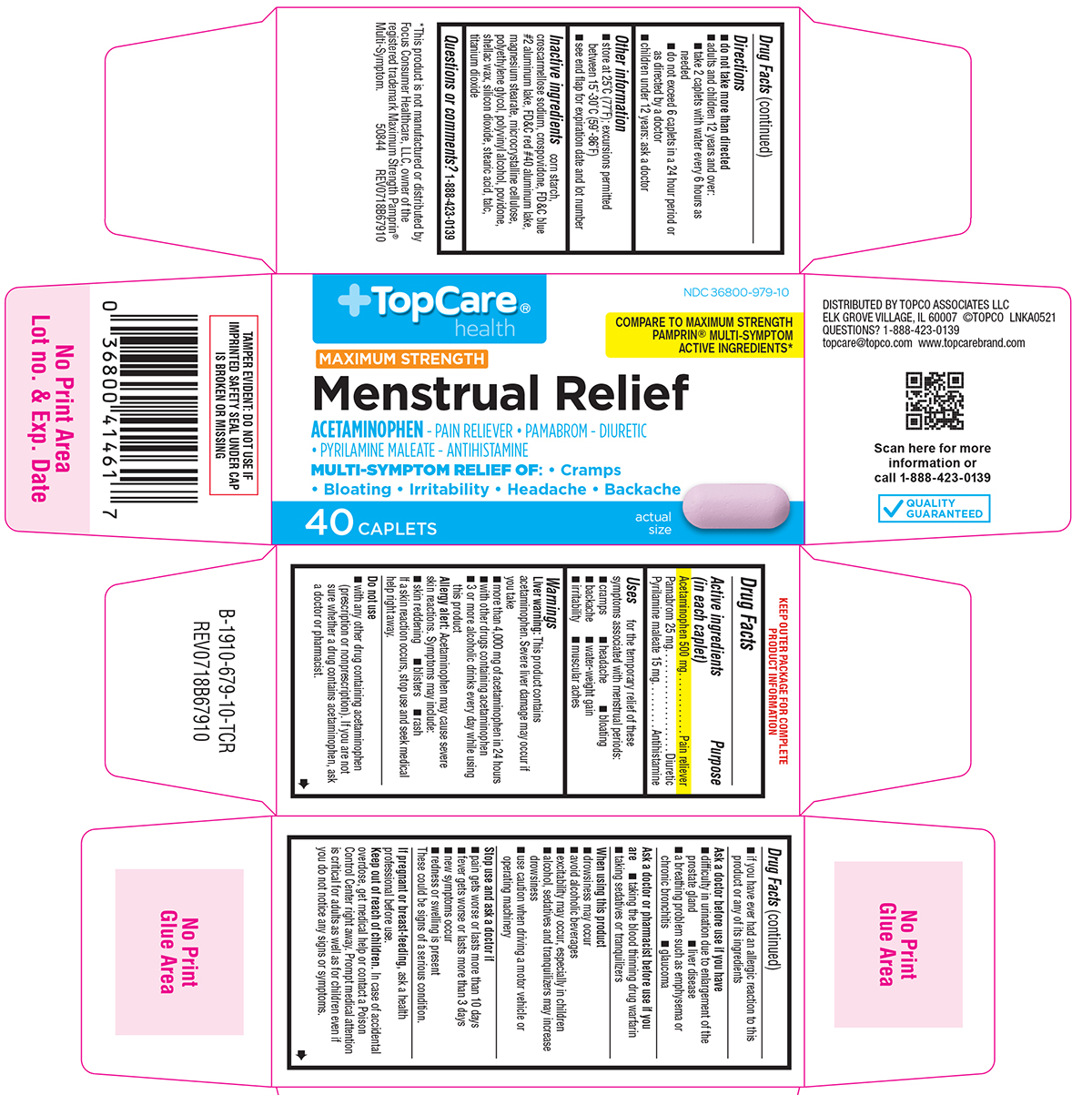
Label: MENSTRUAL RELIEF MAXIMUM STRENGTH- acetaminophen, pamabrom, pyrilamine maleate tablet, film coated
- NDC Code(s): 36800-979-10
- Packager: Topco Associates, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness may occur
- avoid alcoholic beverages
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
TopCare®
healthNDC 36800-979-10
COMPARE TO MAXIMUM STRENGTH
PAMPRIN® MULTI-SYMPTOM ACTIVE
INGREDIENTS*MAXIMUM STRENGTH
Menstrual ReliefACETAMINOPHEN - PAIN RELIEVER • PAMABROM - DIURETIC
• PYRILAMINE MALEATE - ANTIHISTAMINEMULTI-SYMPTOM RELIEF OF: • Cramps
• Bloating • Irritability • Headache • Backache40 CAPLETS
actual
sizeTAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP
IS BROKEN OR MISSINGDISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007 ©TOPCO LNKA0521
QUESTIONS? 1-888-423-0139
topcare@topco.com www.topcarebrand.comQUALITY
GUARANTEED*This product is not manufactured or distributed by
Focus Consumer Healthcare, LLC, owner of the
registered trademark Maximum Strength Pamprin®
Multi-Symptom. 50844 REV0718B67910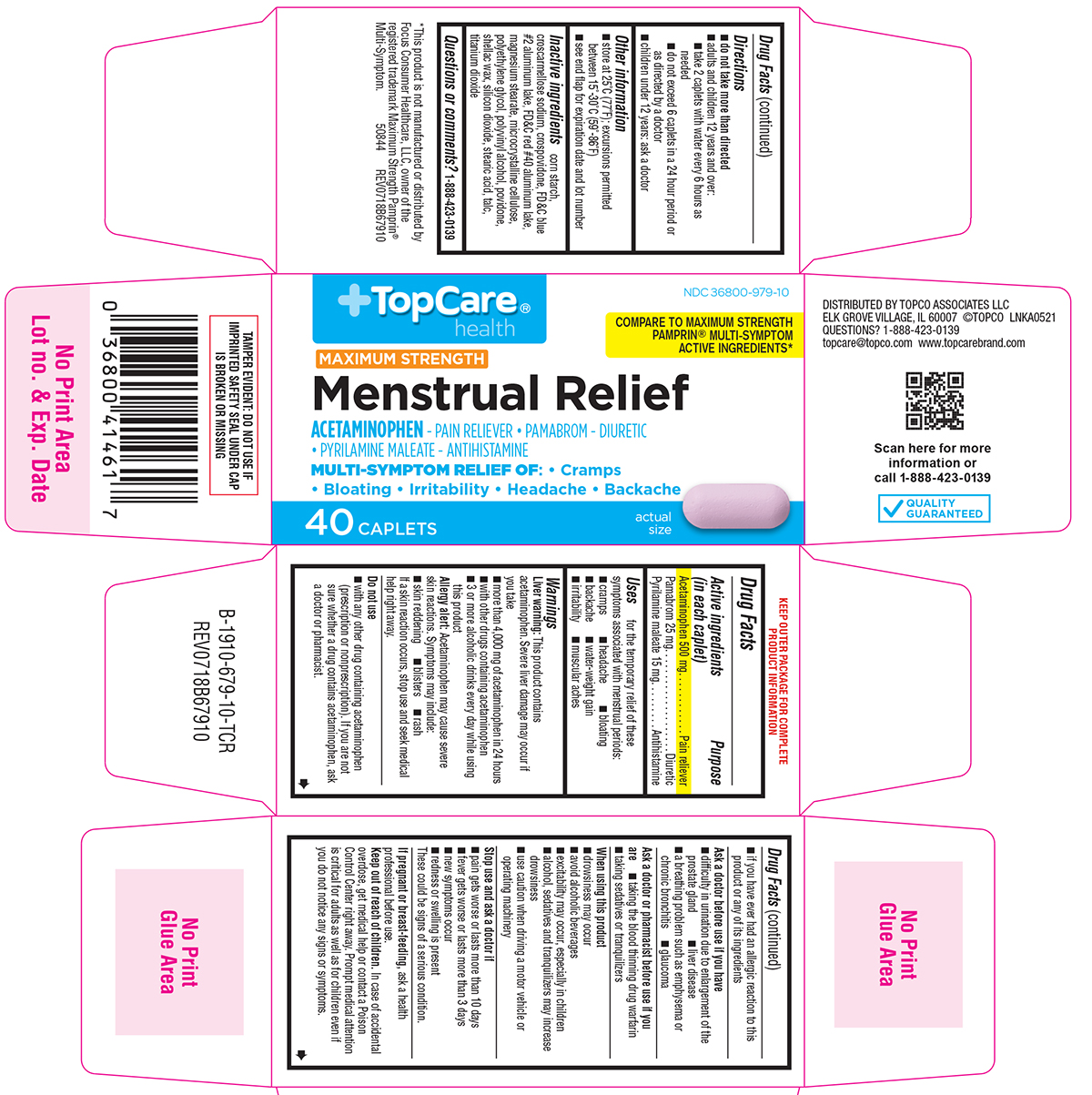
TopCare 44-679
-
INGREDIENTS AND APPEARANCE
MENSTRUAL RELIEF MAXIMUM STRENGTH
acetaminophen, pamabrom, pyrilamine maleate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-979 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg PAMABROM (UNII: UA8U0KJM72) (BROMOTHEOPHYLLINE - UNII:FZG87K1MQ6) PAMABROM 25 mg PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 15 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C BLUE NO. 2 ALUMINUM LAKE (UNII: 4AQJ3LG584) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color purple Score no score Shape OVAL Size 17mm Flavor Imprint Code 44;679 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-979-10 1 in 1 CARTON 01/13/2015 1 40 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/13/2015 Labeler - Topco Associates, LLC (006935977) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(36800-979) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 pack(36800-979) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(36800-979) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 pack(36800-979) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(36800-979)