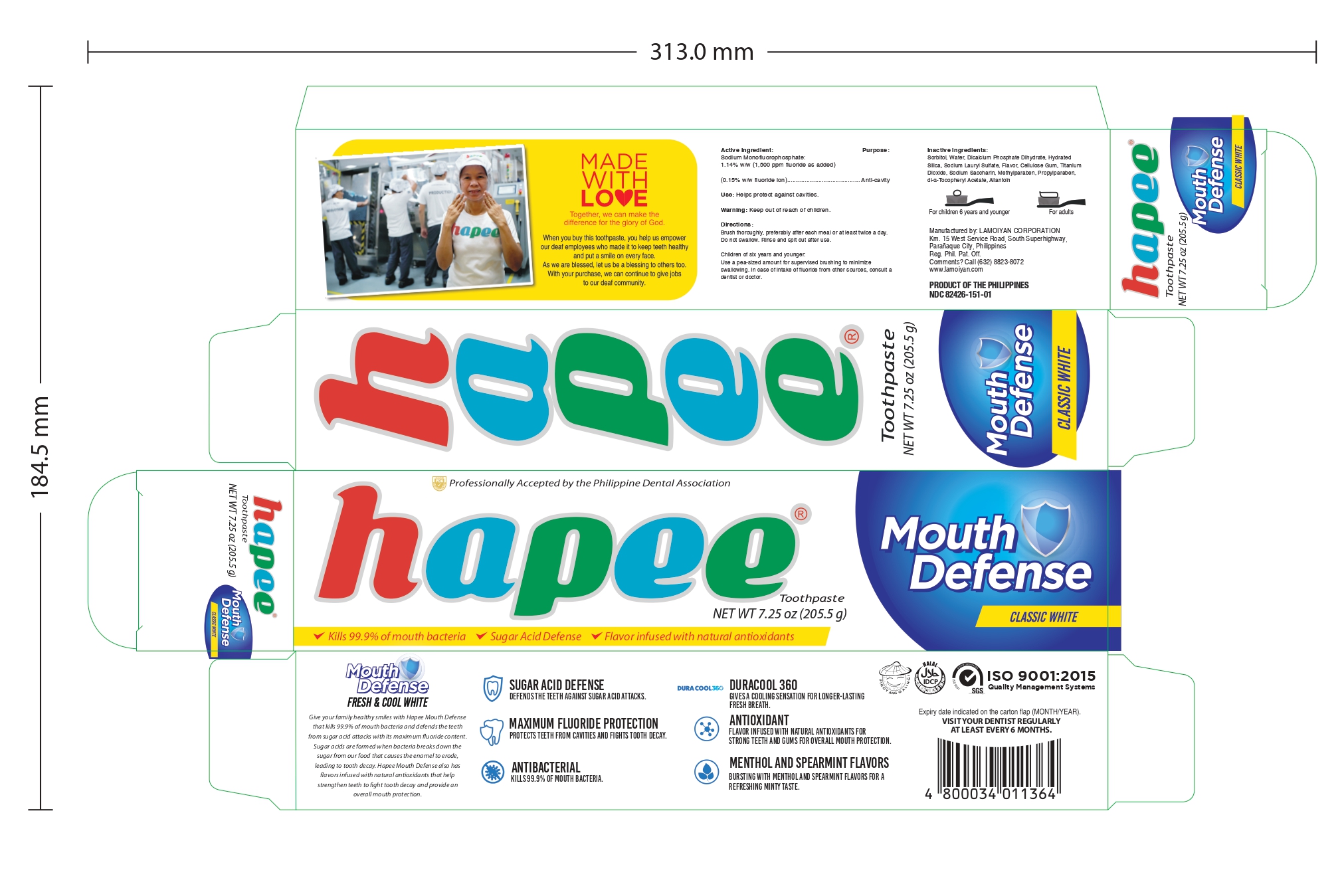
Label: HAPEE EXPLOSIVE MENTHOL RED 150- sodium monofluorophosphate gel, dentifrice
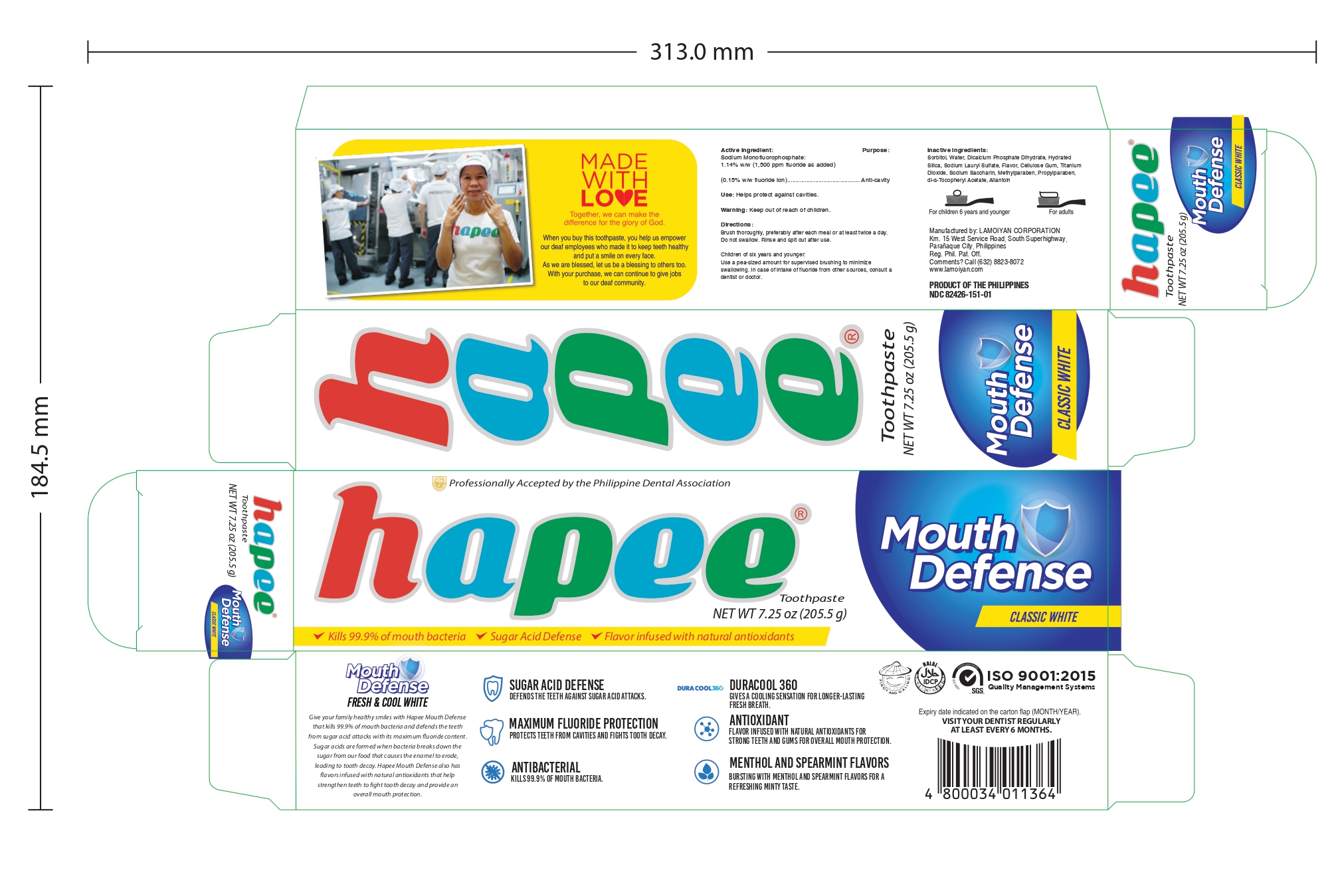
HAPEE FRESH AND COOL WHITE 150- sodium monofluorophosphate gel, dentifrice
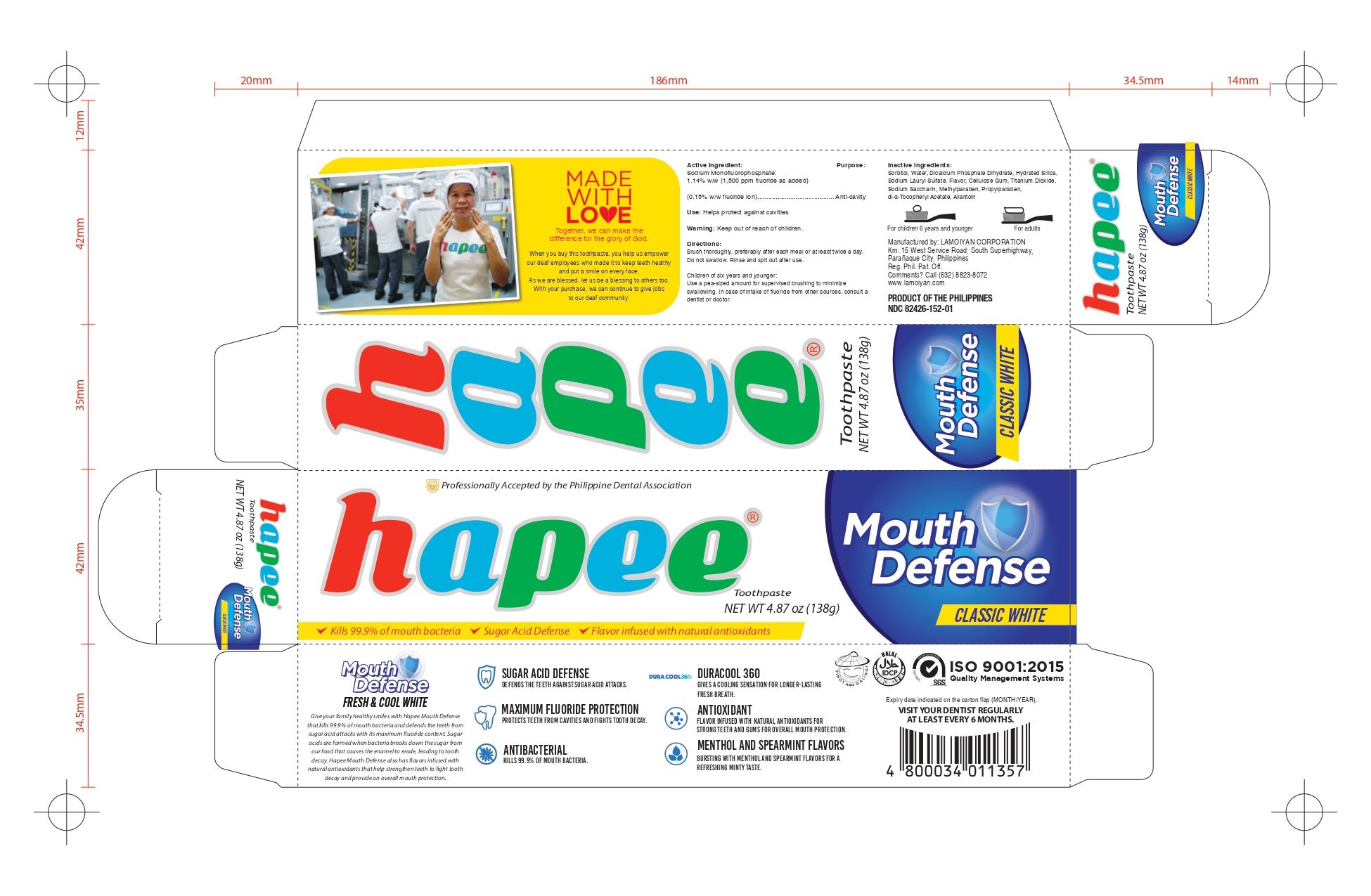
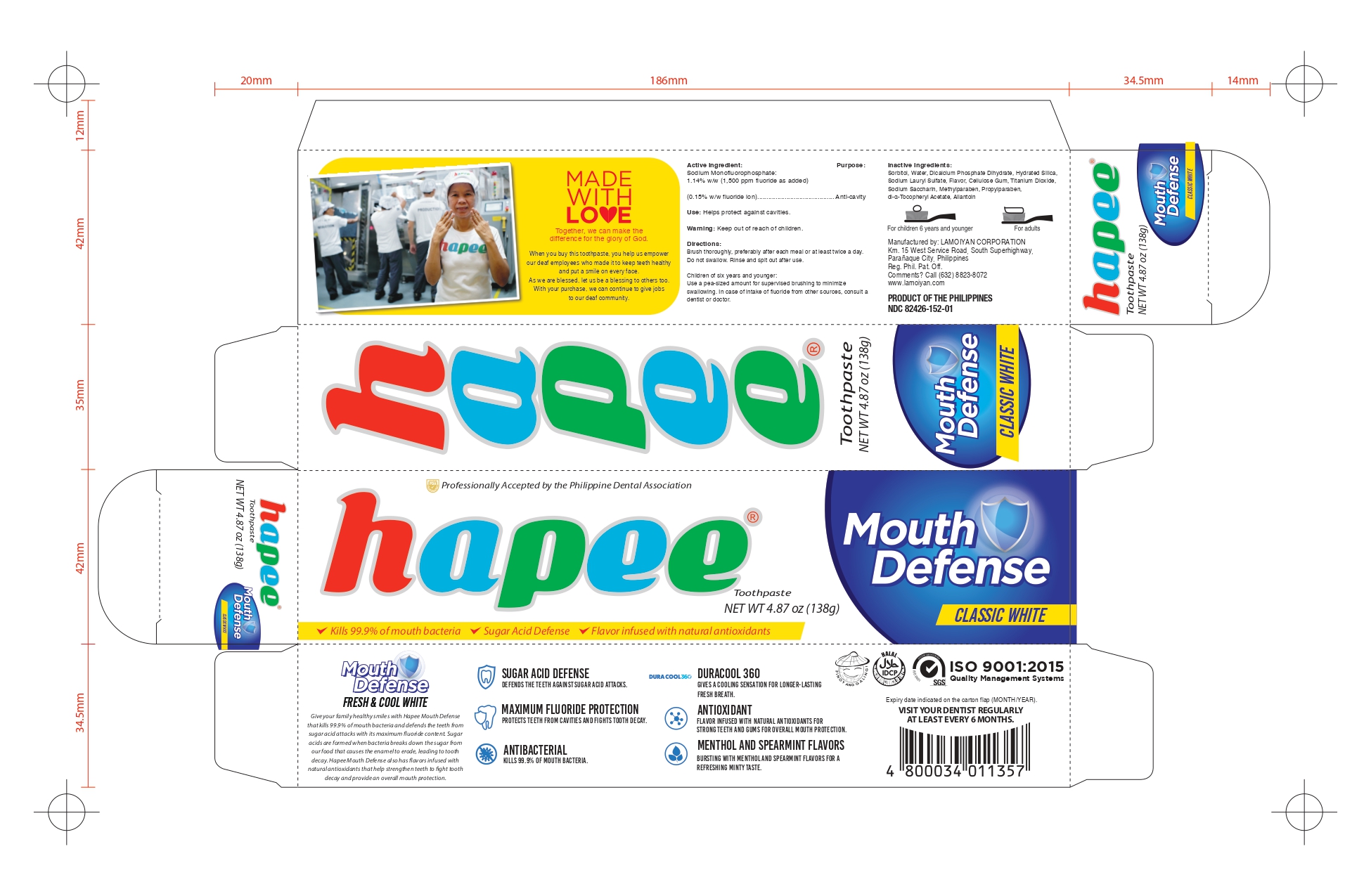
HAPEE FRESH AND COOL WHITE 100- sodium monofluorophosphate gel, dentifrice
HAPEE GUMTECT GUM CARE- sodium monofluorophosphate gel, dentifrice
HAPEE GUMTECT SENSITIVE 100- sodium monofluorophosphate gel, dentifrice
HAPEE FRESH GREEN OUTBURST 100- sodium monofluorophosphate gel, dentifrice
HAPEE FRESH GREEN OUTBURST 150- sodium monofluorophosphate gel, dentifrice
HAPEE OUTRAGEOUS BLUE CHILL 150- sodium monofluorophosphate gel, dentifrice
-
NDC Code(s):
82426-150-01,
82426-151-01,
82426-152-01,
82426-153-01, view more82426-154-01, 82426-155-01, 82426-156-01, 82426-157-01
- Packager: LAMOIYAN CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS SECTION
- Direction
- Warning
- Inactive Ingredients
- Active Ingredient
- Use
- PURPOSE
- PRODUCT SPECIFICATIONS
- HAPEE EXPLOSIVE MENTHOL RED 150
- HAPEE FRESH AND COOL WHITE 150 ML
- HAPEE FRESH AND COOL WHITE 100ML
- HAPEE GUMTECT GUM CARE
- HAPEE GUMTECT SENSITIVE 150
- HAPEE FRESH GREEN OUTBURST 100ML
- HAPEE FRESH GREEN OUTBURST 150 ML
- HAPEE OUTRSGEOUS BLUE CHILL
-
INGREDIENTS AND APPEARANCE
HAPEE EXPLOSIVE MENTHOL RED 150
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-150 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) MICA (UNII: V8A1AW0880) PROPYLPARABEN (UNII: Z8IX2SC1OH) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color red Score Shape Size Flavor MENTHOL (Characteristic, sweet spicy taste) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-150-01 1 in 1 CARTON 05/20/2022 1 189 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 HAPEE FRESH AND COOL WHITE 150
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-151 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) Product Characteristics Color white Score Shape Size Flavor MENTHOL (Methanol and spearmint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-151-01 1 in 1 CARTON 05/27/2022 1 205.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 HAPEE FRESH AND COOL WHITE 100
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-152 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) Product Characteristics Color white Score Shape Size Flavor MENTHOL (Methanol and spearmint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-152-01 1 in 1 CARTON 05/27/2022 1 138 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 HAPEE GUMTECT GUM CARE
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-153 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) SACCHARIN SODIUM (UNII: SB8ZUX40TY) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) O-CYMEN-5-OL (UNII: H41B6Q1I9L) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) ALLANTOIN (UNII: 344S277G0Z) Product Characteristics Color white Score Shape Size Flavor MENTHOL (Rich, rounded spearmint with fresh mentholic peppermint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-153-01 1 in 1 CARTON 05/20/2022 1 151 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 HAPEE GUMTECT SENSITIVE 100
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-154 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.331 g in 100 g POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM HYDROXIDE (UNII: 55X04QC32I) SUCRALOSE (UNII: 96K6UQ3ZD4) O-CYMEN-5-OL (UNII: H41B6Q1I9L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALLANTOIN (UNII: 344S277G0Z) Product Characteristics Color white Score Shape Size Flavor MENTHOL (Characteristic, sweet minty taste) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-154-01 1 in 1 CARTON 05/27/2022 1 133 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/20/2022 HAPEE FRESH GREEN OUTBURST 100
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-155 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) CELLULOSE SODIUM PHOSPHATE (UNII: E6S1NJ4Y5Q) MICA (UNII: V8A1AW0880) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color green Score Shape Size Flavor MENTHOL (Sweet spicy taste) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-155-01 1 in 1 CARTON 05/27/2022 1 128 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 HAPEE FRESH GREEN OUTBURST 150
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-156 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) CELLULOSE SODIUM PHOSPHATE (UNII: E6S1NJ4Y5Q) MICA (UNII: V8A1AW0880) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color green Score Shape Size Flavor MENTHOL (Sweet spicy taste) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-156-01 1 in 1 CARTON 05/27/2022 1 192 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 HAPEE OUTRAGEOUS BLUE CHILL 150
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82426-157 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) MICA (UNII: V8A1AW0880) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color blue Score Shape Size Flavor MENTHOL (sweet spicy taste) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82426-157-01 1 in 1 CARTON 05/27/2022 1 192 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 05/20/2022 Labeler - LAMOIYAN CORPORATION (718765928) Establishment Name Address ID/FEI Business Operations LAMOIYAN CORPORATION 718765928 manufacture(82426-150, 82426-151, 82426-152, 82426-153, 82426-154, 82426-155, 82426-156, 82426-157)