HAPEE EXPLOSIVE MENTHOL RED 150- sodium monofluorophosphate gel, dentifrice
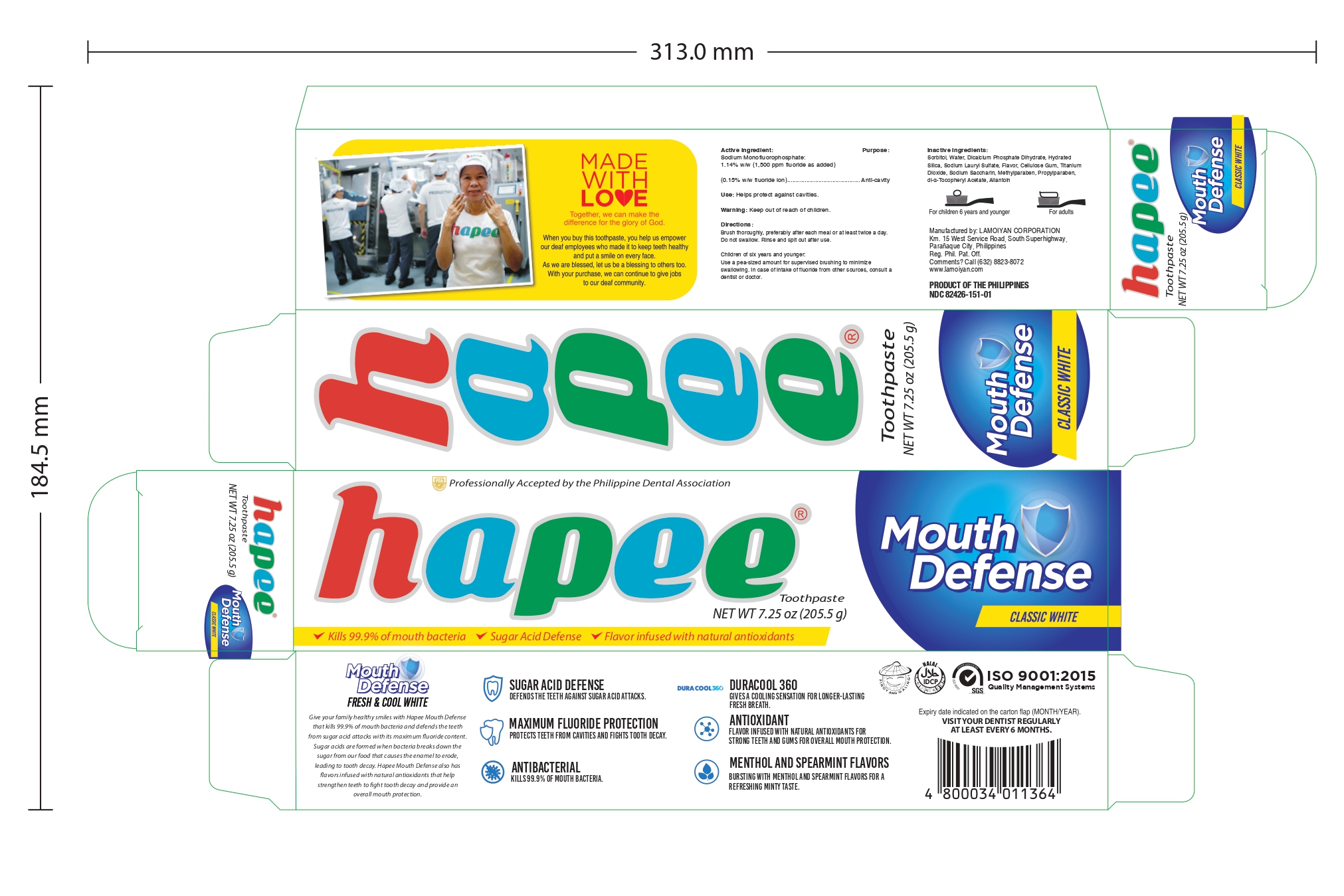
HAPEE FRESH AND COOL WHITE 150- sodium monofluorophosphate gel, dentifrice
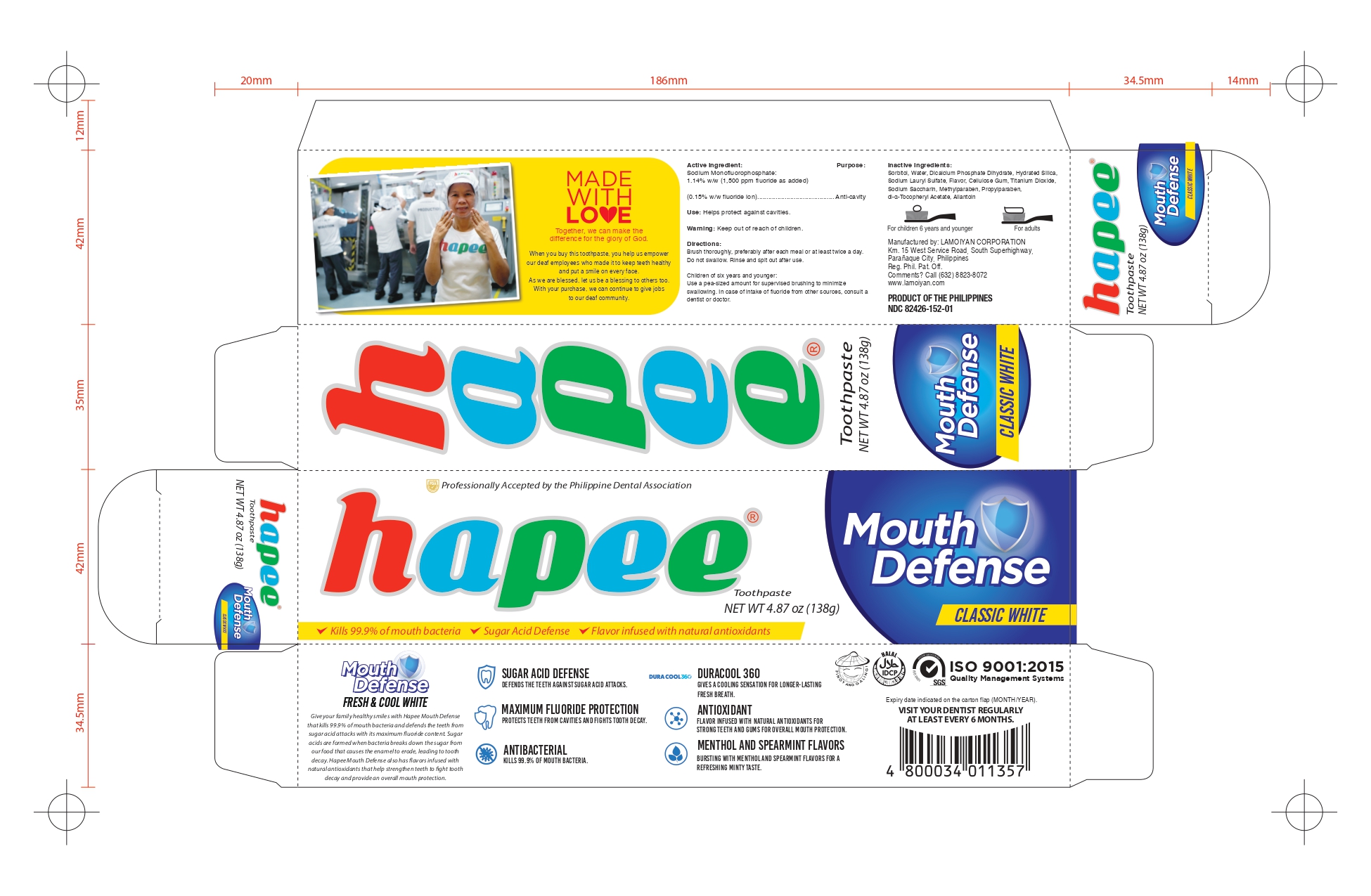
HAPEE FRESH AND COOL WHITE 100- sodium monofluorophosphate gel, dentifrice
HAPEE GUMTECT GUM CARE- sodium monofluorophosphate gel, dentifrice
HAPEE GUMTECT SENSITIVE 100- sodium monofluorophosphate gel, dentifrice
HAPEE FRESH GREEN OUTBURST 100- sodium monofluorophosphate gel, dentifrice
HAPEE FRESH GREEN OUTBURST 150- sodium monofluorophosphate gel, dentifrice
HAPEE OUTRAGEOUS BLUE CHILL 150- sodium monofluorophosphate gel, dentifrice
LAMOIYAN CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
WARNINGS AND PRECAUTIONS SECTION
Children of six years and younger: Use a pea-sized amount for supervised brushing to minimize swallowing. In case of intake of fluoride from other sources, consult a dentist or doctor.
Direction
Brush thoroughly, preferably after each meal or at least twice a day. Do not swallow. Rinse and spit out after use.
Warning
Keep out of reach of children
Inactive Ingredients
Sorbitol, Water, Hydrated Silica, Sodium Lauryl Sulfate, Flavor, PEG-12, Cellulose Gum, Sodium Saccharin, Methylparaben, Mica, Propylparaben, FD&C Red No.40
Active Ingredient
Sodium Monofluorophosphate:
1.14% w/w (1,500 ppm fluoride as added)
(0.15% w/w fluoride ion)
Use
Helps protect against cavities
HAPEE EXPLOSIVE MENTHOL RED 150

HAPEE FRESH AND COOL WHITE 150 ML
HAPEE FRESH AND COOL WHITE 100ML
HAPEE GUMTECT GUM CARE

HAPEE GUMTECT SENSITIVE 150

HAPEE FRESH GREEN OUTBURST 100ML

HAPEE FRESH GREEN OUTBURST 150 ML

HAPEE OUTRSGEOUS BLUE CHILL








