Label: BORAQUA EYE WASH- water solution
- NDC Code(s): 69666-871-04
- Packager: Genuine Drugs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Active ingredient
- Uses
- Warnings
-
Directions
Remove contact lenses before using.
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after use.

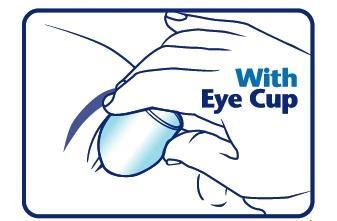
When using an eye cup
- rinse the cup with Pure-aid Eye Wash immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Pure-aid Eye Wash Solution and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse cup with clean water after each use
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BORAQUA EYE WASH
water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69666-871 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 99.05 mL in 100 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYAMINOPROPYL BIGUANIDE (UNII: 322U039GMF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69666-871-04 1 in 1 BOX 04/20/2015 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 04/20/2015 Labeler - Genuine Drugs (079610378)


