Label: PRECAINE- topical anesthetic gel gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 10866-0062-1 - Packager: Pascal Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE FORMS & STRENGTHS
-
DOSAGE & ADMINISTRATION
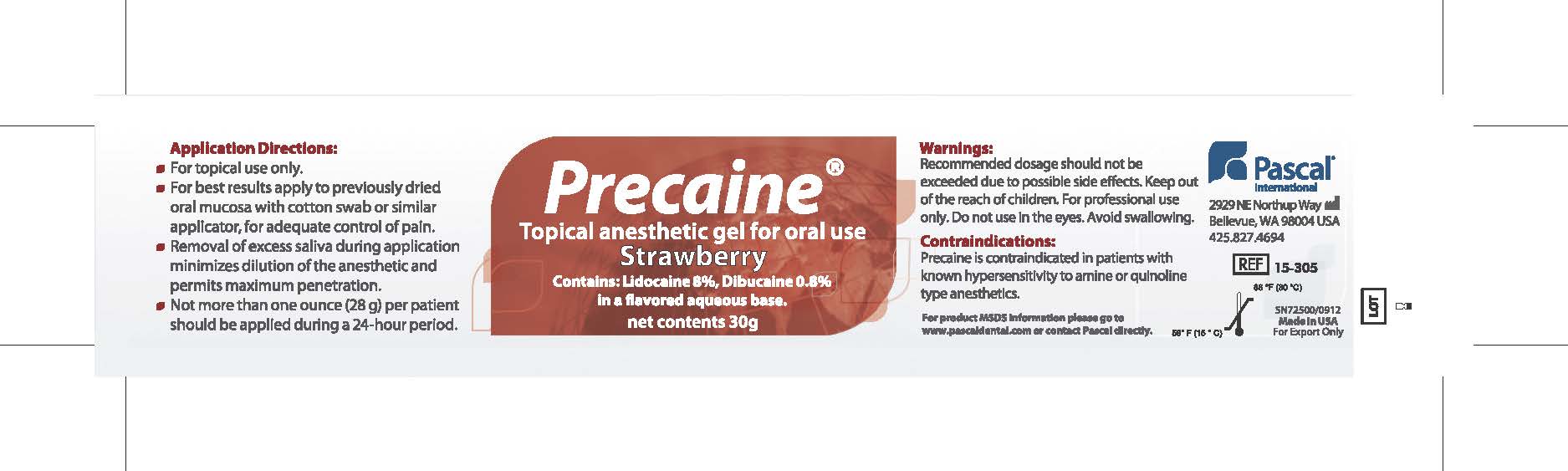
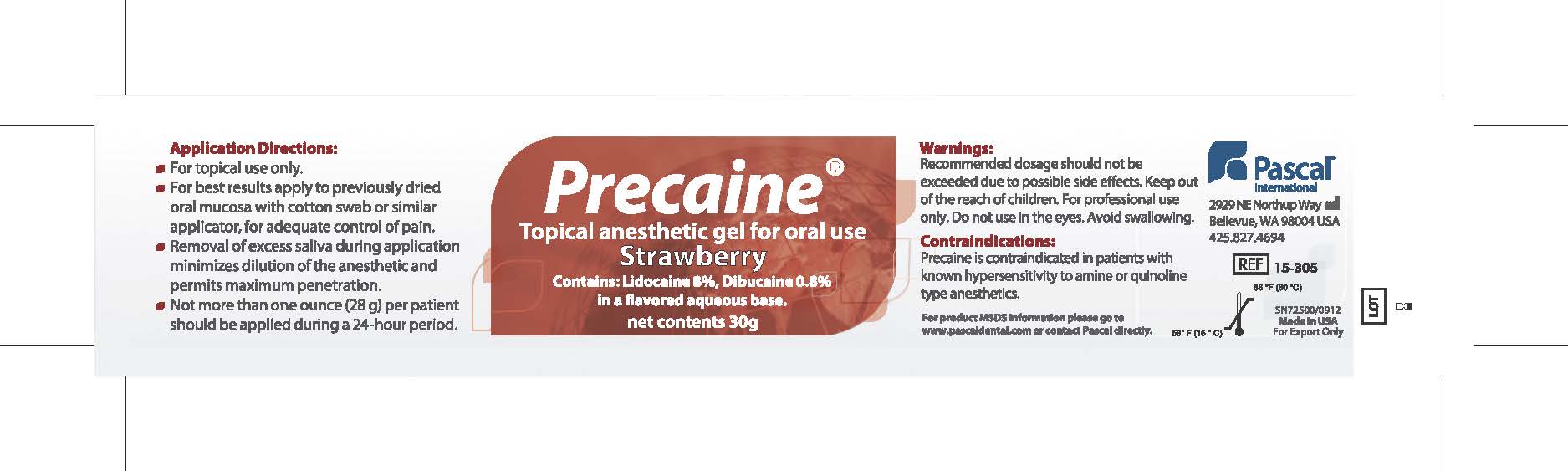
Application Directions:
- For topical use only
- For best results apply to previously dried oral mucosa with cotton swab or similar applicator for adequate control of pain.
- Removal of excess saliva during application minimizes dilution of the anesthetic and permits maximum penetration.
- Not more than one ounce (28g) per patient should be applied during a 24 hour period.
- WARNINGS
- CONTRAINDICATIONS
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRECAINE
topical anesthetic gel gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10866-0062 Route of Administration BUCCAL, TOPICAL, DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 80 mg in 1 g DIBUCAINE (UNII: L6JW2TJG99) (DIBUCAINE - UNII:L6JW2TJG99) DIBUCAINE 8 mg in 1 g Product Characteristics Color red Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10866-0062-1 30 g in 1 JAR; Type 0: Not a Combination Product 08/01/2000 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/01/2000 Labeler - Pascal Company, Inc. (009260217) Registrant - Alexia Petropoulos (009260217) Establishment Name Address ID/FEI Business Operations Pascal Company, Inc. 009260217 manufacture(10866-0062)