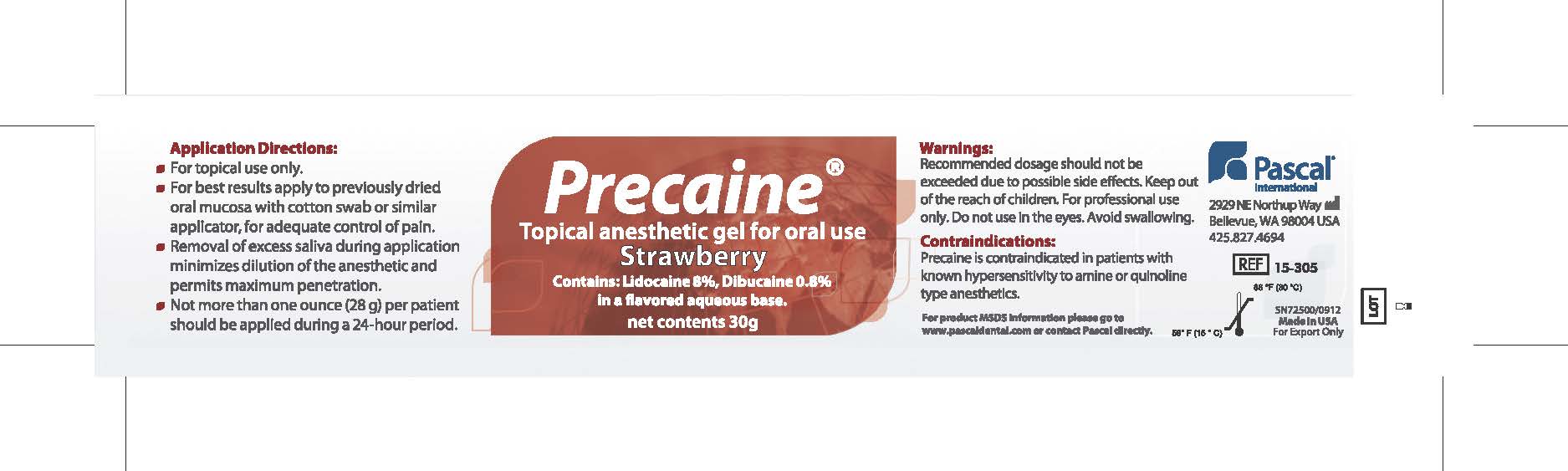
Application Directions:
- For topical use only
- For best results apply to previously dried oral mucosa with cotton swab or similar applicator for adequate control of pain.
- Removal of excess saliva during application minimizes dilution of the anesthetic and permits maximum penetration.
- Not more than one ounce (28g) per patient should be applied during a 24 hour period.
Recommended dosage should not be exceeded due to possible side effects. Keep out of the reach of children. For professional use only. Do not use in the eyes. Avoid swallowing.