Label: A AND D ORIGINAL- lanolin and petrolatum ointment
- NDC Code(s): 11523-0096-3, 11523-0096-7
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
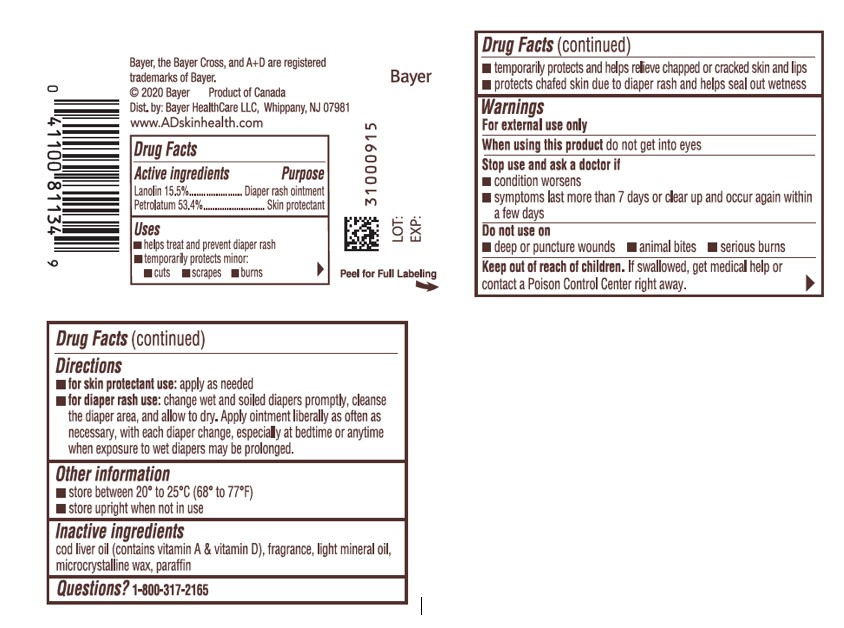
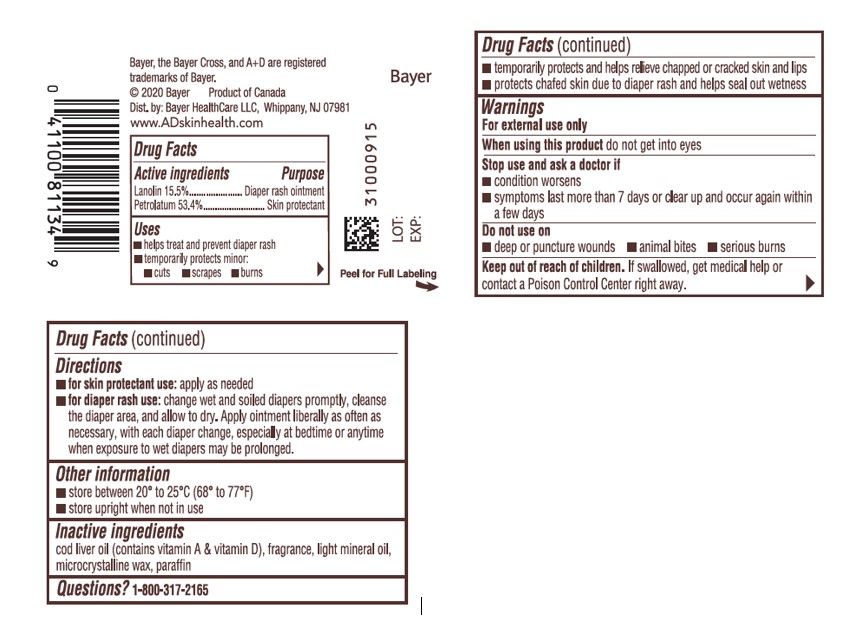
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 454g Jar Label
-
INGREDIENTS AND APPEARANCE
A AND D ORIGINAL
lanolin and petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-0096 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 136.4 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 469.9 mg in 1 g Inactive Ingredients Ingredient Name Strength COD LIVER OIL (UNII: BBL281NWFG) LIGHT MINERAL OIL (UNII: N6K5787QVP) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PARAFFIN (UNII: I9O0E3H2ZE) Product Characteristics Color orange (Unctuous amber mass) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-0096-7 340 g in 1 JAR; Type 0: Not a Combination Product 02/13/2023 09/08/2023 2 NDC:11523-0096-3 454 g in 1 JAR; Type 0: Not a Combination Product 02/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/25/2009 Labeler - Bayer HealthCare LLC. (112117283)