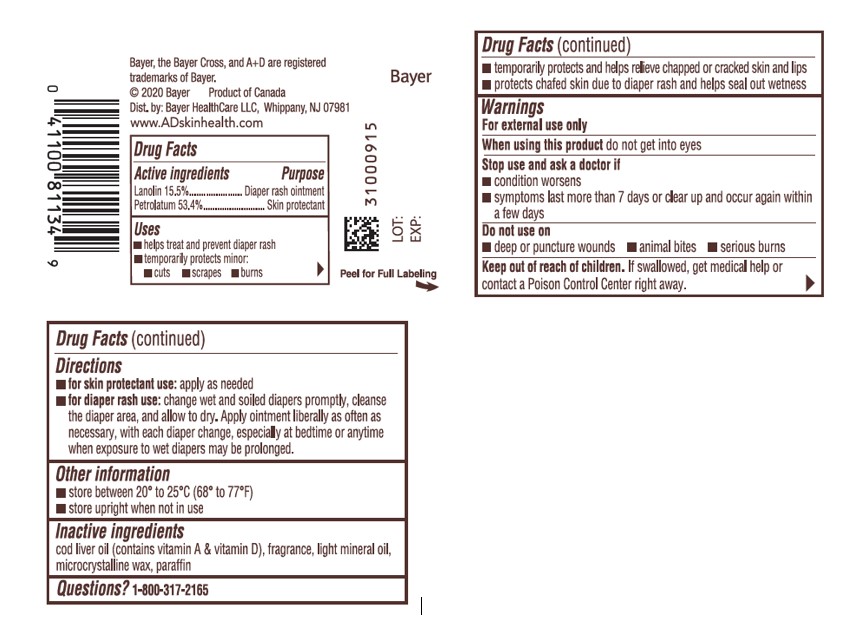
| Active ingredients | Purpose |
| Lanolin 15.5% | Diaper rash ointment |
| Petrolatum 53.4% | Skin Protectant |
Uses
- helps treat and prevent diaper rash
- temporarily protects minor:
- temporarily protects and helps relieve chapped, chafed or cracked skin and lips
- protect chafed skin due to diaper rash and helps seal out wetness
Warnings
For external use only
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Do not use on
- deep or puncture wounds .
- animal bites
- serious burns .
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
for skin protectant use apply as needed
-
for diaper rash use:
- change wet and soiled diapers promptly
- cleanse the diaper area, and allow to dry
- apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
Other information
- store between 20°C to 25 °C ( 68° to 77°F)
- store upright when not in use
Inactive Ingredients
cod liver oil ( contains vitamin A & vitamin D), fragrance, light mineral oil, microcrystalline wax, paraffin
Bayer, the Bayer Cross, and A+D are registered trademarks of Bayer
2020 Bayer Product of Canada
Dist. by: Bayer Healthcare LLC, Whippany, NJ 07981
www.ADskinhealth.com
PRINCIPAL DISPLAY PANEL - 454g Jar Label
A+D
®
Prevent
Original Ointment
DIAPER RASH OINTMENT
+ SKIN PROTECTANT
Helps prevent &
treat diaper rash
Forms a protective barrier
to help seal out wetness
Paraben Free
Dye Free
Phthalate Free
PEDIATRICIAN RECOMMENDED
NET WT 1LB (454g)

Bayer HealthCare LLC.

