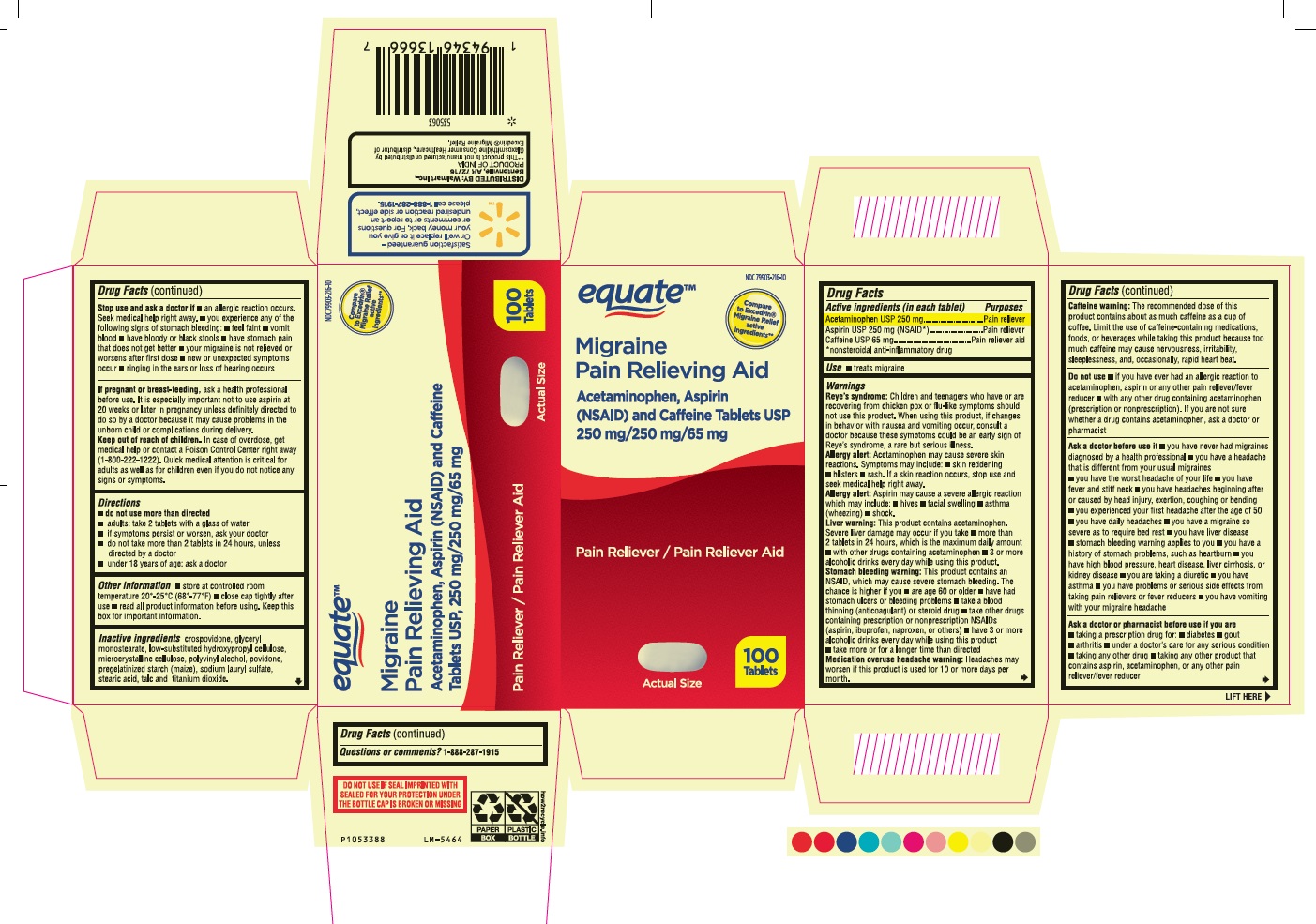
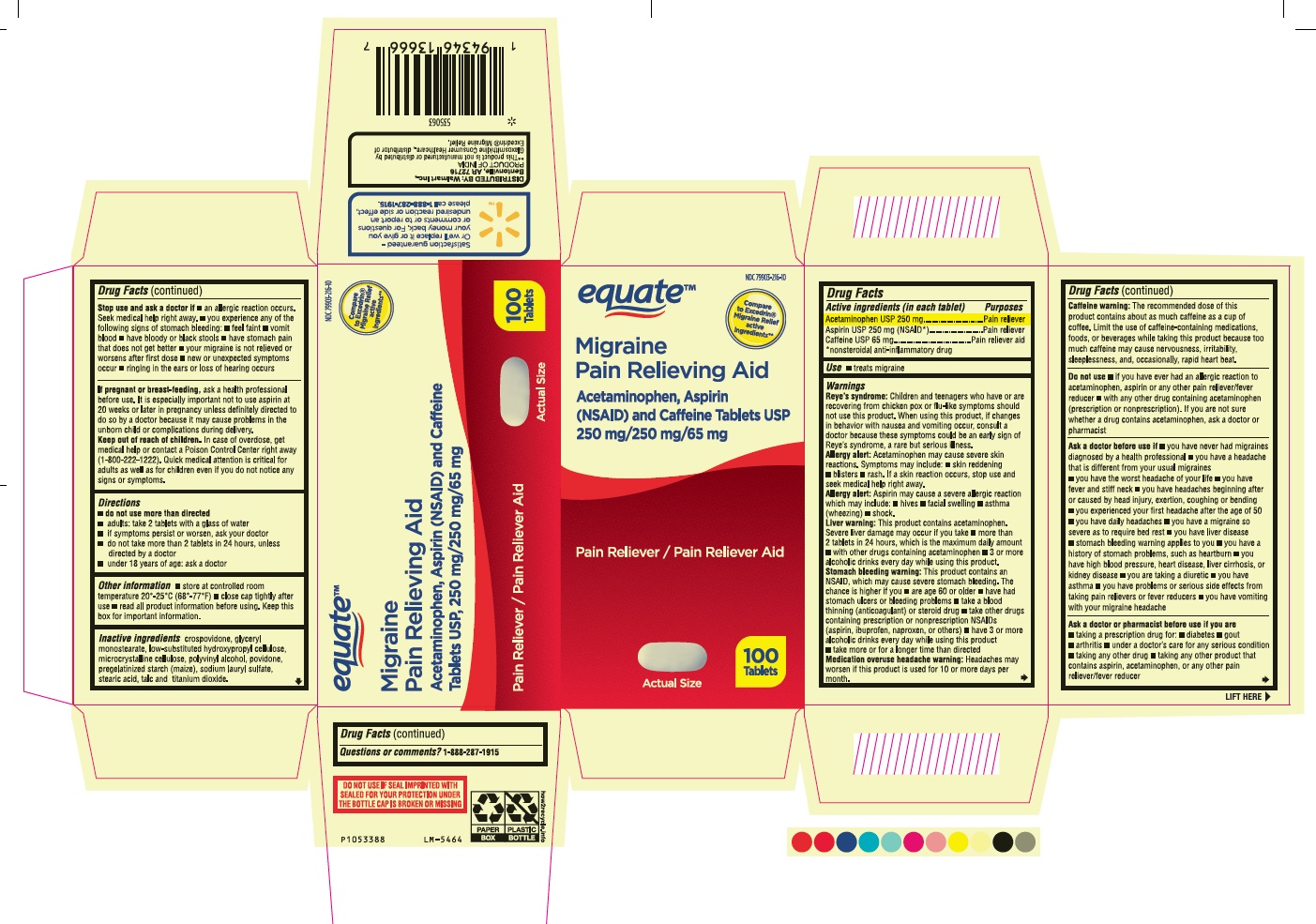
Label: ACETAMINOPHEN ASPIRIN AND CAFFEINE- acetaminophen, aspirin and caffeine tablet, film coated
- NDC Code(s): 79903-216-10
- Packager: WALMART INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purposes
- Use
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include: • skin reddening • blisters • rash. If a skin reaction occurs, stop use and seek medical help right away.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: • hives • facial swelling • asthma (wheezing) • shock.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 2 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older • have had stomach ulcers or bleeding problems • take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product.
- take more or for a longer time than directed
Medication overuse headache warning: Headaches may worsen if this product is used for 10 or more days per month.
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
- Do not use
-
Ask a doctor before use if
- you have never had migraines diagnosed by a health professional
- you have a headache that is different from your usual migraines
- you have the worst headache of your life
- you have fever and stiff neck
- you have headaches beginning after or caused by head injury, exertion, coughing or bending
- you experienced your first headache after the age of 50
- you have daily headaches
- you have a migraine so severe as to require bed rest
- you have liver disease
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have asthma
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have vomiting with your migraine headache
- Ask a doctor or pharmacist before use if you are
-
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- your migraine is not relieved or worsens after first dose
- new or unexpected symptoms occur
- ringing in the ears or loss of hearing occurs
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/250 mg/65 mg Container Label - 100 Tablets
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/250 mg/65 mg Container Carton Label - 100 Tablets
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN ASPIRIN AND CAFFEINE
acetaminophen, aspirin and caffeine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-216 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K30 (UNII: U725QWY32X) POVIDONE K90 (UNII: RDH86HJV5Z) STARCH, CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White to off-White) Score no score Shape CAPSULE (biconvex) Size 18mm Flavor Imprint Code T;57 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-216-10 1 in 1 CARTON 02/28/2024 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211695 02/28/2024 Labeler - WALMART INC. (051957769) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650918514 ANALYSIS(79903-216) , MANUFACTURE(79903-216)