Label: TRICARE PRENATAL 2-PART DAILY PRENATAL VITAMIN SYSTEM- 18 essential vitamins and minerals, ultra pure dha kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 67112-201-60, 67112-502-60, 67112-802-60 - Packager: Medecor Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 13, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
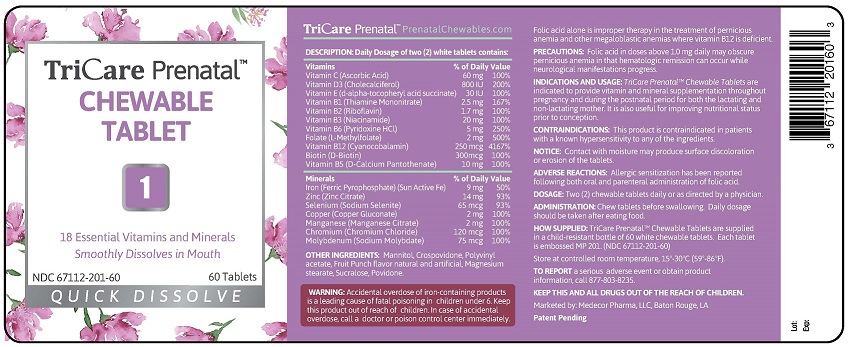
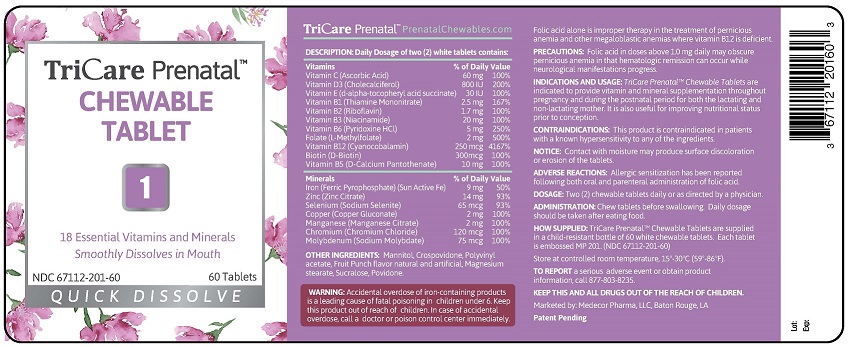
STATEMENT OF IDENTITY
INDICATIONS AND USAGE: TriCare Prenatal™ Chewable
Tablets with Twist-off DHA Softgels are indicated to provide
vitamin and mineral supplementation throughout
pregnancy and during the postnatal period for both the
lactating and non-lactating mother. It is also useful for
improving nutritional status prior to conception.
CONTRAINDICATIONS: This product is contraindicated in
patients with a known hypersensitivity to any of the
ingredients.
NOTICE: Contact with moisture may produce surface
discoloration or erosion of the tablets and twist-off softgels.
ADVERSE REACTIONS: Allergic sensitization has been
reported following both oral and parenteral administration
of folic acid.
DOSAGE: Two (2) chewable tablets and two (2) twist-off
softgels daily or as directed by a physician.
ADMINISTRATION: Chew tablets before swallowing. Twist
small end off from softgel and squeeze contents directly into
mouth or into juice to drink. Daily dosage should be taken
with food.
HOW SUPPLIED: Child-resistant, blister pack of two (2) white
tablets and two (2) purple twist-off softgels.
Each tablet is embossed MP 201.
Each Twist-off softgel is marked MP 802.
(NDC 67112-502-60)
Store at controlled room temperature, 15º-30ºC (59º-86ºF).
TO REPORT a serious adverse event or obtain product
information, call 877-803-8235.
KEEP THIS AND ALL DRUGS OUT OF THE REACH
OF CHILDREN.
Marketed by: Medecor Pharma, LLC, Baton Rouge, LA
Patent Pending
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRICARE PRENATAL 2-PART DAILY PRENATAL VITAMIN SYSTEM
18 essential vitamins and minerals, ultra pure dha kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67112-502 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67112-502-60 1 in 1 BOX 05/10/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 0 BOTTLE 1 Part 2 1 BOTTLE 1 Part 1 of 2 TRICARE PRENATAL TWIST-OFF DHA SOFT QUICK DISSOLVE
dha, epa, other omega-3s capsule, gelatin coatedProduct Information Item Code (Source) NHRIC:67112-802 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 150 ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 37.5 OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3 FATTY ACIDS 75 Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) COCHINEAL (UNII: TZ8Z31B35M) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) COCONUT OIL (UNII: Q9L0O73W7L) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TOCOPHEROL (UNII: R0ZB2556P8) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ORANGE OIL (UNII: AKN3KSD11B) Product Characteristics Color purple Score no score Shape CAPSULE Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:67112-802-60 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 05/10/2016 Part 2 of 2 TRICARE PRENATAL QUICK DISSOLVE
18 essential vitamins and minerals tablet, chewableProduct Information Item Code (Source) NHRIC:67112-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 30 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL SUCCINATE, D- 15 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.25 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN .85 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 10 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 2.5 mg LEVOMEFOLIC ACID (UNII: 8S95DH25XC) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN .125 mg BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN .150 mg CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 5 mg FERRIC PYROPHOSPHATE (UNII: QK8899250F) (FERRIC CATION - UNII:91O4LML611) FERRIC CATION 4.5 mg ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 7 mg SODIUM SELENITE (UNII: HIW548RQ3W) (SELENITE ION - UNII:KXO0259XJ1) SODIUM SELENITE .0325 mg COPPER GLUCONATE (UNII: RV823G6G67) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg MANGANESE CITRATE DECAHYDRATE (UNII: 4Z2OA6A13N) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE CITRATE DECAHYDRATE 1 mg CHROMIC CHLORIDE CR-51 (UNII: U4M5Y13FGM) (CHROMIUM CR-51 - UNII:9QAU17N705) CHROMIC CHLORIDE CR-51 .060 mg SODIUM MOLYBDATE (UNII: 948QAQ08I1) (MOLYBDATE ION - UNII:O0L10E6352) SODIUM MOLYBDATE .0375 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) CROSPOVIDONE (UNII: 2S7830E561) POLYVINYL ACETATE (UNII: 32K497ZK2U) MAGNESIUM STEARATE (UNII: 70097M6I30) SUCRALOSE (UNII: 96K6UQ3ZD4) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score no score Shape OVAL Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:67112-201-60 1 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 03/21/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/10/2016 Labeler - Medecor Pharma, LLC (830621046)