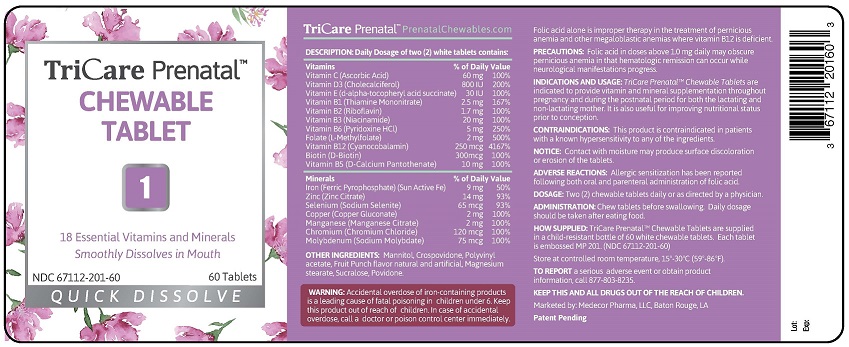
INDICATIONS AND USAGE: TriCare Prenatal™ Chewable
Tablets with Twist-off DHA Softgels are indicated to provide
vitamin and mineral supplementation throughout
pregnancy and during the postnatal period for both the
lactating and non-lactating mother. It is also useful for
improving nutritional status prior to conception.
CONTRAINDICATIONS: This product is contraindicated in
patients with a known hypersensitivity to any of the
ingredients.
NOTICE: Contact with moisture may produce surface
discoloration or erosion of the tablets and twist-off softgels.
ADVERSE REACTIONS: Allergic sensitization has been
reported following both oral and parenteral administration
of folic acid.
DOSAGE: Two (2) chewable tablets and two (2) twist-off
softgels daily or as directed by a physician.
ADMINISTRATION: Chew tablets before swallowing. Twist
small end off from softgel and squeeze contents directly into
mouth or into juice to drink. Daily dosage should be taken
with food.
HOW SUPPLIED: Child-resistant, blister pack of two (2) white
tablets and two (2) purple twist-off softgels.
Each tablet is embossed MP 201.
Each Twist-off softgel is marked MP 802.
(NDC 67112-502-60)
Store at controlled room temperature, 15º-30ºC (59º-86ºF).
TO REPORT a serious adverse event or obtain product
information, call 877-803-8235.
KEEP THIS AND ALL DRUGS OUT OF THE REACH
OF CHILDREN.
Marketed by: Medecor Pharma, LLC, Baton Rouge, LA
Patent Pending