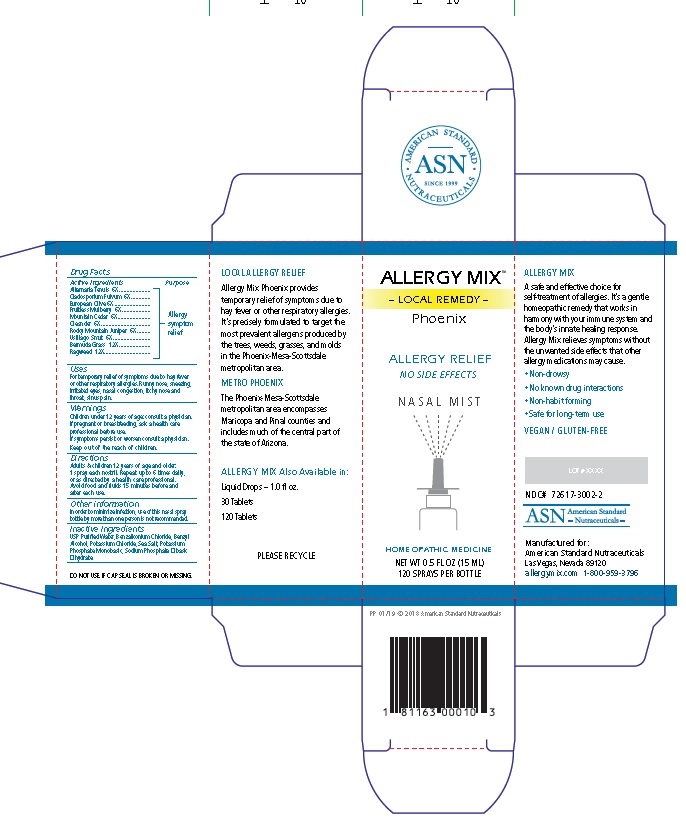
Label: ALLERGY MIX PHOENIX- local remedy phoenix spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 72617-3002-2 - Packager: ASN
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 28, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
Principal Display Package
AMERICAN STANDARD
• ASN •
SINCE 1999
NUTRACEUTICALSALLERGY MIX™
– LOCAL REMEDY –
PhoenixALLERGY RELIEF
NO SIDE EFFECTS
N A S A L M I S T
HOMEOPATHIC MEDICINE
NET WT 0.5 FL OZ (15 ML)
120 SPRAYS PER BOTTLE
PP 01/19 © 2018 American Standard Nutraceuticals
1 81163 00010 3
LOCAL ALLERGY RELIEF
Allergy Mix Phoenix provides
temporary relief of symptoms due to
hay fever or other respiratory allergies.
It’s precisely formulated to target the
most prevalent allergens produced by
the trees, weeds, grasses, and molds
in the Phoenix-Mesa-Scottsdale
metropolitan area.
METRO PHOENIX
The Phoenix-Mesa-Scottsdale
metropolitan area encompasses
Maricopa and Pinal counties and
includes much of the central part of
the state of Arizona.
ALLERGY MIX Also Available in:
Liquid Drops – 1.0 fl oz.
30 Tablets
120 Tablets
PLEASE RECYCLE
DO NOT USE IF CAP SEAL IS BROKEN OR MISSING.
ALLERGY MIX
A safe and effective choice for
self-treatment of allergies. It’s a gentle
homeopathic remedy that works in
harmony with your immune system and
the body’s innate healing response.
Allergy Mix relieves symptoms without
the unwanted side effects that other
allergy medications may cause.• Non-drowsy
• No known drug interactions
• Non-habit forming
• Safe for long-term use
VEGAN / GLUTEN-FREE
LOT # XXXX
NDC# 72617-3002-2
ASN American Standard
- Nutraceuticals -Manufactured for:
American Standard Nutraceuticals
Las Vegas, Nevada 89120
allergymix.com 1-800-959-3796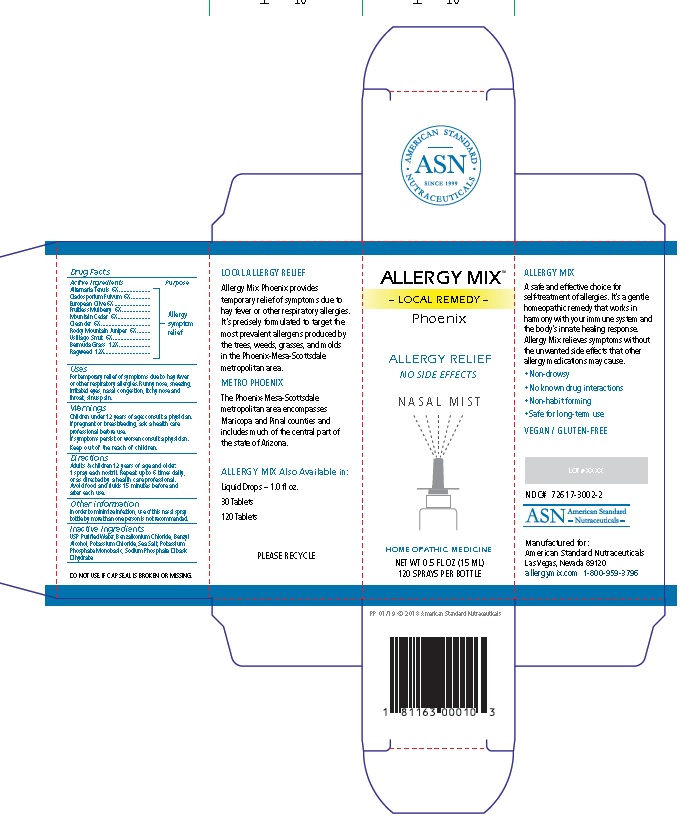
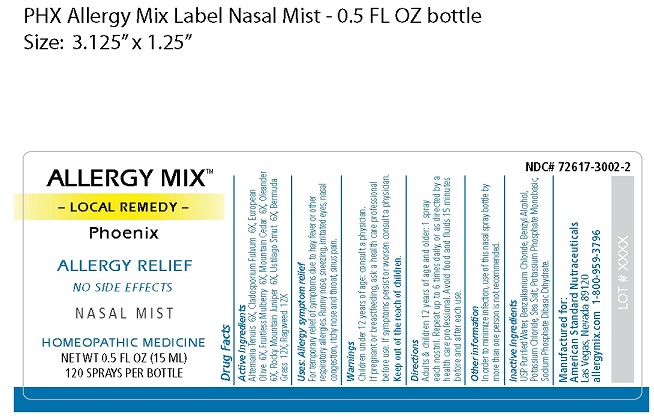
↓Bottle Label↓
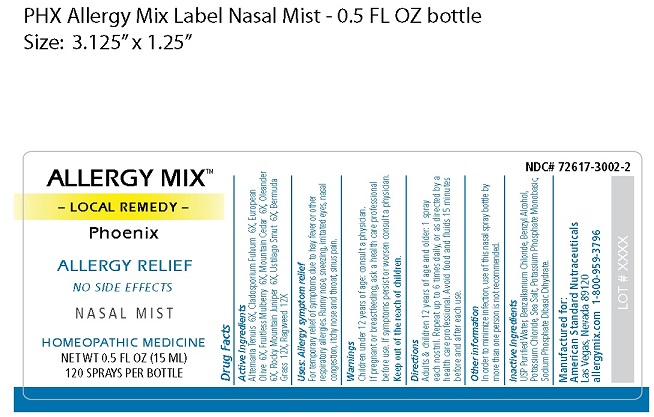
res
-
INGREDIENTS AND APPEARANCE
ALLERGY MIX PHOENIX
local remedy phoenix sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72617-3002 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALTERNARIA ALTERNATA (UNII: 52B29REC7H) (ALTERNARIA ALTERNATA - UNII:52B29REC7H) ALTERNARIA ALTERNATA 6 [hp_X] in 15 mL PASSALORA FULVA (UNII: HR6H5057CO) (PASSALORA FULVA - UNII:HR6H5057CO) PASSALORA FULVA 6 [hp_X] in 15 mL OLEA EUROPAEA FLOWER (UNII: 498M34P1VZ) (OLEA EUROPAEA FLOWER - UNII:498M34P1VZ) OLEA EUROPAEA FLOWER 6 [hp_X] in 15 mL WHITE MULBERRY (UNII: MN25R0HH5A) (WHITE MULBERRY - UNII:MN25R0HH5A) WHITE MULBERRY 6 [hp_X] in 15 mL JUNIPERUS ASHEI POLLEN (UNII: 544F8MEY0Y) (JUNIPERUS ASHEI POLLEN - UNII:544F8MEY0Y) JUNIPERUS ASHEI POLLEN 6 [hp_X] in 15 mL NERIUM OLEANDER FLOWER (UNII: WO4WVF1WVM) (NERIUM OLEANDER FLOWER - UNII:WO4WVF1WVM) NERIUM OLEANDER FLOWER 6 [hp_X] in 15 mL JUNIPERUS SCOPULORUM POLLEN (UNII: 0G82TT8ZFY) (JUNIPERUS SCOPULORUM POLLEN - UNII:0G82TT8ZFY) JUNIPERUS SCOPULORUM POLLEN 6 [hp_X] in 15 mL USTILAGO MAYDIS (UNII: 4K7Z7K7SWG) (USTILAGO MAYDIS - UNII:4K7Z7K7SWG) USTILAGO MAYDIS 6 [hp_X] in 15 mL CYNODON DACTYLON (UNII: 2Q8MR21NHK) (CYNODON DACTYLON - UNII:2Q8MR21NHK) CYNODON DACTYLON 12 [hp_X] in 15 mL AMBROSIA ARTEMISIIFOLIA (UNII: 9W34L2CQ9A) (AMBROSIA ARTEMISIIFOLIA - UNII:9W34L2CQ9A) AMBROSIA ARTEMISIIFOLIA 12 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SEA SALT (UNII: 87GE52P74G) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) SODIUM PHOSPHATE DIBASIC DIHYDRATE (UNII: 94255I6E2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72617-3002-2 1 in 1 PACKAGE 01/23/2019 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2019 Labeler - ASN (012050067) Registrant - ASN (012050067)