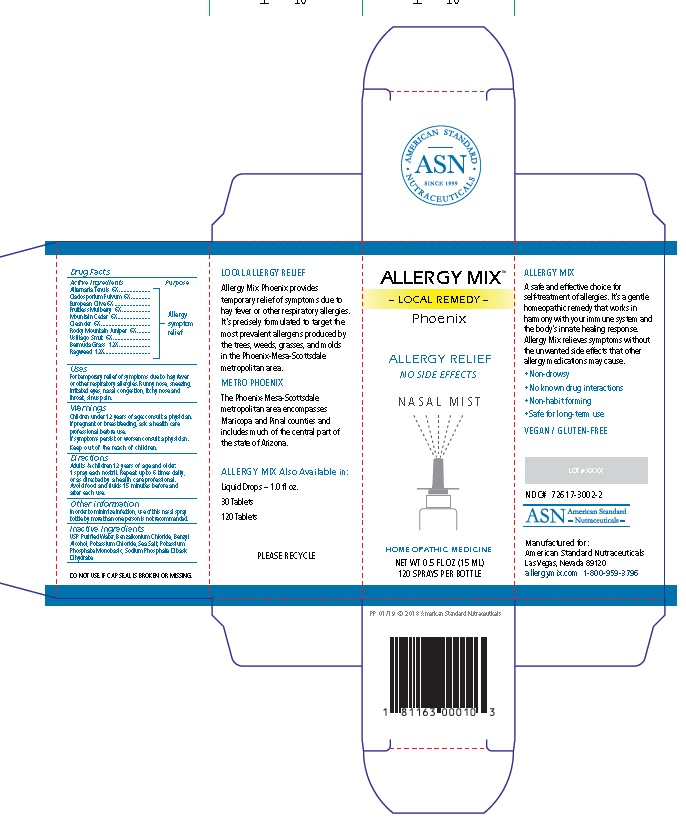
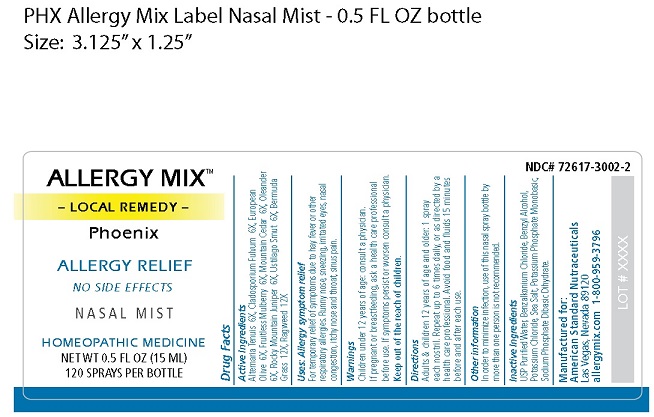
Active Ingredients
Alternaria Tenuis 6X
Cladosporium Fulvum 6X
European Olive 6X
Fruitless Mulberry 6X
Mountain Cedar 6X
Oleander 6X
Rocky Mountain Juniper 6X
Ustilago Smut 6X
Bermuda Grass 12X
Ragweed 12X
Uses
For temporary relief of symptoms due to hay fever
or other respiratory allergies. Runny nose, sneezing,
irritated eyes, nasal congestion, itchy nose and
throat, sinus pain.
Warnings
Children under 12 years of age: consult a physician.
If pregnant or breastfeeding, ask a health care
professional before use.
If symptoms persist or worsen consult a physician.
Directions
Adults & children 12 years of age and older:
1 spray each nostril. Repeat up to 6 times daily,
or as directed by a health care professional.
Avoid food and fluids 15 minutes before and
after each use.
Other information
In order to minimize infection, use of this nasal spray
bottle by more than one person is not recommended.
Inactive Ingredients
USP Purified Water, Benzalkonium Chloride, Benzyl
Alcohol, Potassium Chloride, Sea Salt, Potassium
Phosphate Monobasic, Sodium Phosphate Dibasic
Dihydrate.
Principal Display Package
AMERICAN STANDARD
• ASN •
SINCE 1999
NUTRACEUTICALS
ALLERGY MIX™
– LOCAL REMEDY –
Phoenix
ALLERGY RELIEF
NO SIDE EFFECTS
N A S A L M I S T
HOMEOPATHIC MEDICINE
NET WT 0.5 FL OZ (15 ML)
120 SPRAYS PER BOTTLE
PP 01/19 © 2018 American Standard Nutraceuticals
1 81163 00010 3
LOCAL ALLERGY RELIEF
Allergy Mix Phoenix provides
temporary relief of symptoms due to
hay fever or other respiratory allergies.
It’s precisely formulated to target the
most prevalent allergens produced by
the trees, weeds, grasses, and molds
in the Phoenix-Mesa-Scottsdale
metropolitan area.
METRO PHOENIX
The Phoenix-Mesa-Scottsdale
metropolitan area encompasses
Maricopa and Pinal counties and
includes much of the central part of
the state of Arizona.
ALLERGY MIX Also Available in:
Liquid Drops – 1.0 fl oz.
30 Tablets
120 Tablets
PLEASE RECYCLE
DO NOT USE IF CAP SEAL IS BROKEN OR MISSING.
ALLERGY MIX
A safe and effective choice for
self-treatment of allergies. It’s a gentle
homeopathic remedy that works in
harmony with your immune system and
the body’s innate healing response.
Allergy Mix relieves symptoms without
the unwanted side effects that other
allergy medications may cause.
• Non-drowsy
• No known drug interactions
• Non-habit forming
• Safe for long-term use
VEGAN / GLUTEN-FREE
LOT # XXXX
NDC# 72617-3002-2
ASN American Standard
- Nutraceuticals -
Manufactured for:
American Standard Nutraceuticals
Las Vegas, Nevada 89120
allergymix.com 1-800-959-3796
↓Bottle Label↓
res