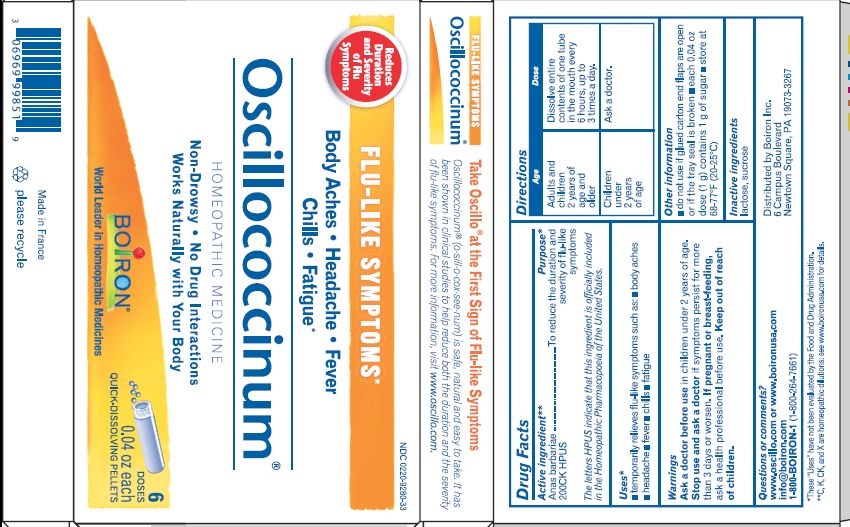
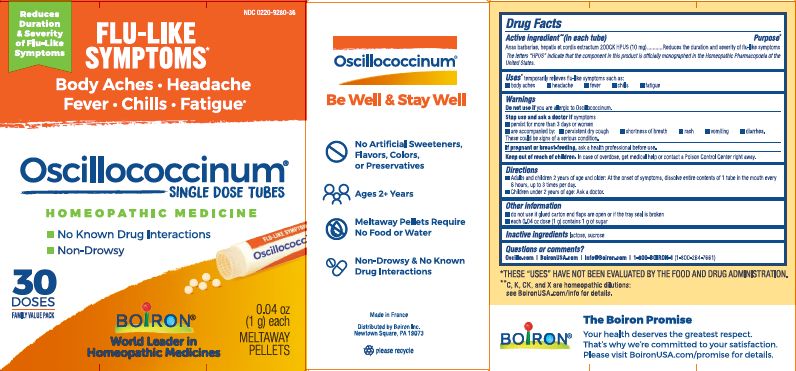
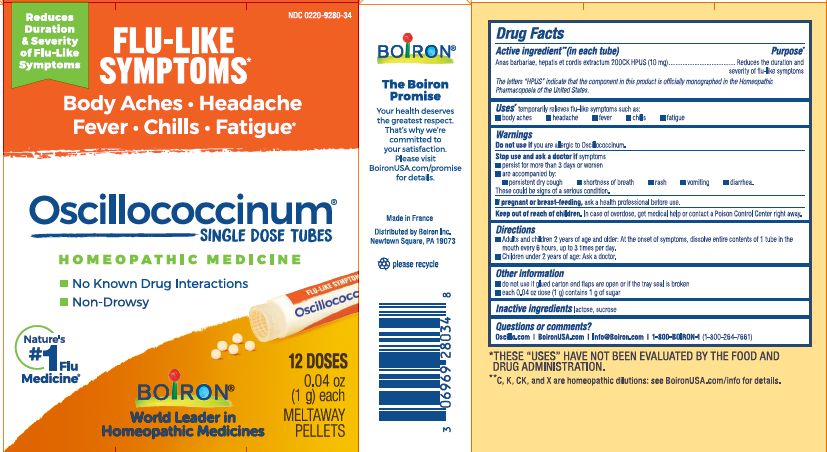
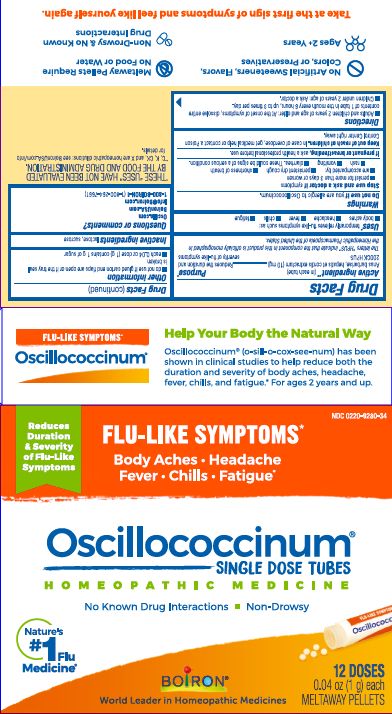
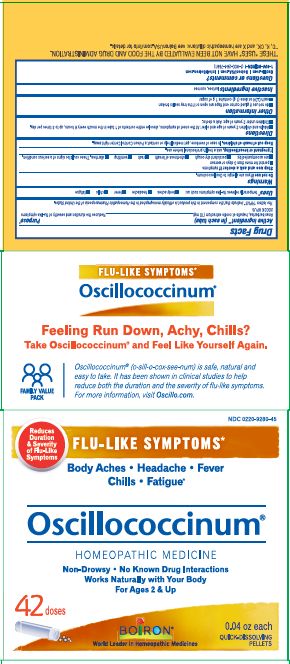
Label: OSCILLOCOCCINUM- cairina moschata heart/liver autolysate pellet
-
NDC Code(s):
0220-9280-31,
0220-9280-32,
0220-9280-33,
0220-9280-34, view more0220-9280-35, 0220-9280-36, 0220-9280-37, 0220-9280-38, 0220-9280-44, 0220-9280-45, 0220-9280-96
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
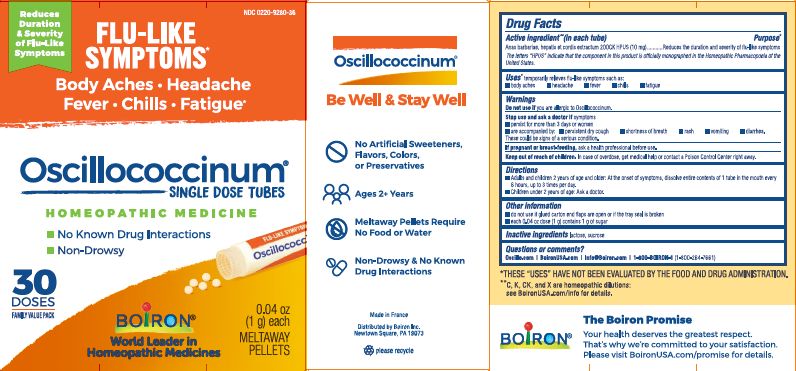
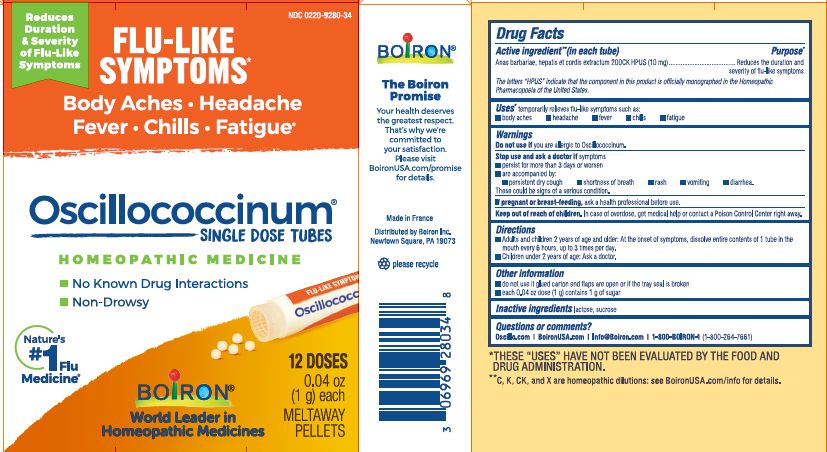
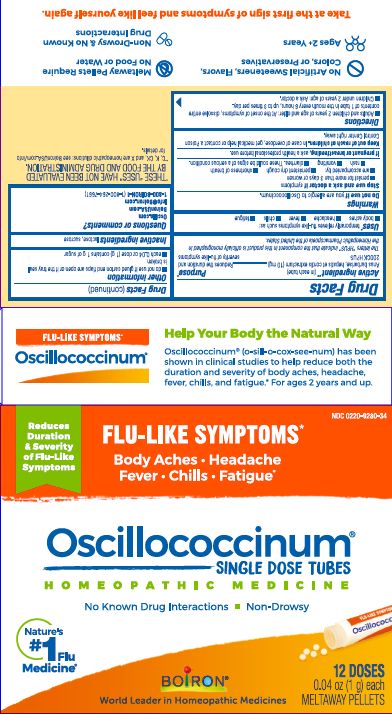
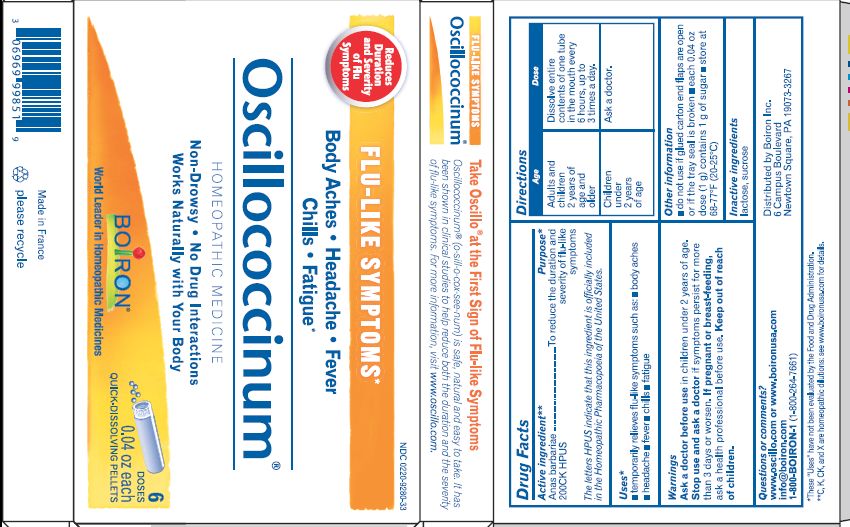
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
SPL UNCLASSIFIED SECTION
do not use if glued carton end flaps are open or if the tray seal is broken
each 0.04 oz dose (1g) contains 1g of sugar
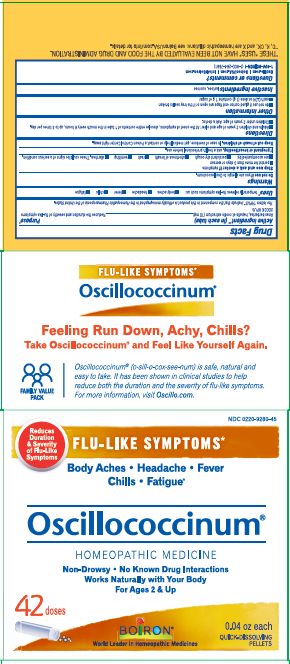
0.04 oz (1 g) each
Meltaway Pellets
Body Aches, Headache, Fever, Chills, Fatigue*
Flu-like Symptoms*
Non-Drowsy No Known Drug Interactions
Works Naturally with your Body
3 Dose
6 Dose
12 Dose
24 Dose
30 Dose36 Dose
42 Dose*THESE “USES” HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OSCILLOCOCCINUM
cairina moschata heart/liver autolysate pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9280 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9280-32 3 in 1 CARTON 08/01/1998 1 200 in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0220-9280-31 200 in 1 TUBE; Type 0: Not a Combination Product 08/01/1998 12/31/2012 3 NDC:0220-9280-33 6 in 1 CARTON 08/01/1998 3 200 in 1 TUBE; Type 0: Not a Combination Product 4 NDC:0220-9280-37 9 in 1 CARTON 08/01/1998 12/31/2014 4 200 in 1 TUBE; Type 0: Not a Combination Product 5 NDC:0220-9280-34 12 in 1 CARTON 08/01/1998 5 200 in 1 TUBE; Type 0: Not a Combination Product 6 NDC:0220-9280-35 18 in 1 CARTON 08/01/1998 12/31/2012 6 200 in 1 TUBE; Type 0: Not a Combination Product 7 NDC:0220-9280-38 27 in 1 CARTON 08/01/1998 12/31/2014 7 200 in 1 TUBE; Type 0: Not a Combination Product 8 NDC:0220-9280-36 30 in 1 CARTON 08/01/1998 8 200 in 1 TUBE; Type 0: Not a Combination Product 9 NDC:0220-9280-44 24 in 1 CARTON 07/29/2020 9 200 in 1 TUBE; Type 0: Not a Combination Product 10 NDC:0220-9280-45 42 in 1 CARTON 05/06/2022 10 200 in 1 TUBE; Type 0: Not a Combination Product 11 NDC:0220-9280-96 36 in 1 CARTON 08/01/2022 11 200 in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/01/1998 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9280)