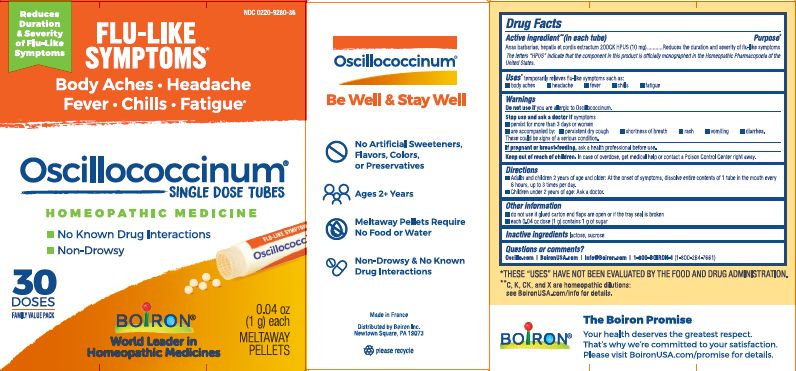
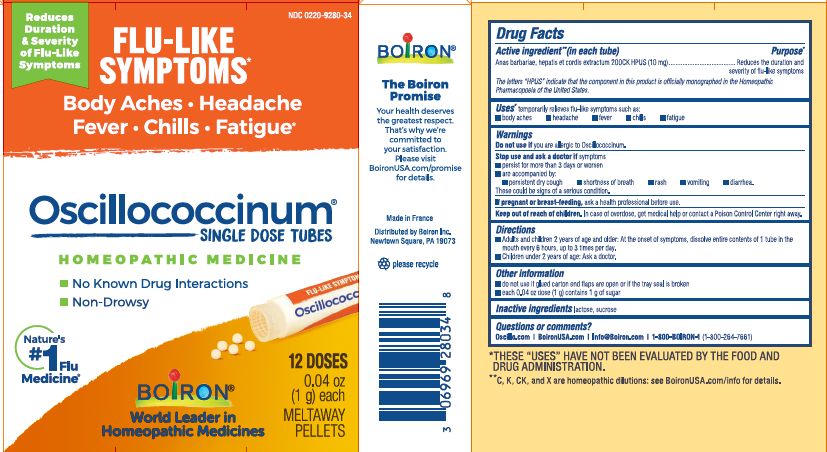
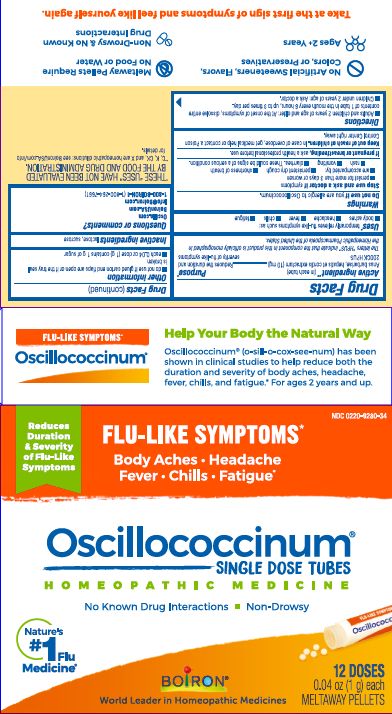
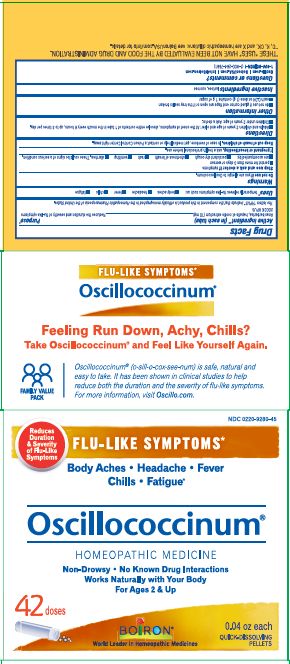
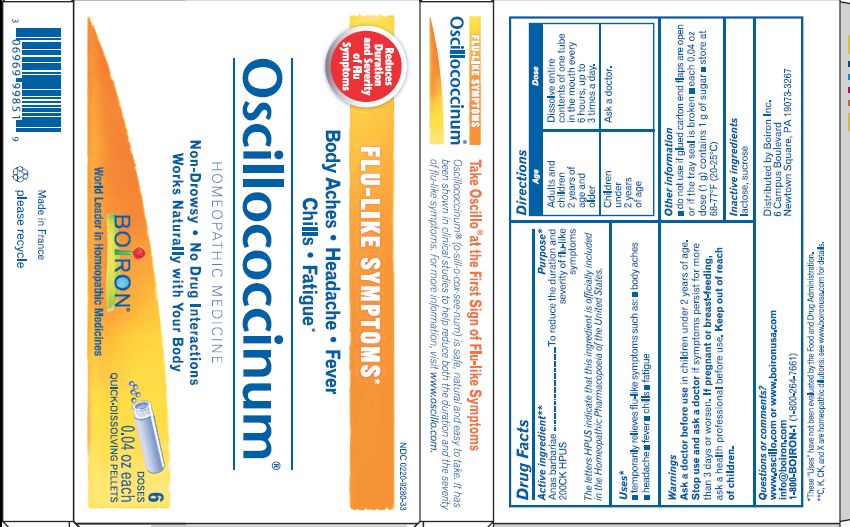
Active Ingredient** (in each tube)
Anas barbariae, hepatis et cordis extractum (10 mg) 200CK HPUS
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Purpose*
Anas barbariae, hepatis et cordis extractum 200CK HPUS ..... Reduces the duration and severity of flu-like symptoms
Stop use and ask a doctor if symptoms
- persist for more than 3 days or worsen
- are accompanied by:
- persistent dry cough
- shortness of breath
- rash
- vomiting
- diarrhea.
These could be signs of a serious condition.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Adults and children 2 years of age and older: At the onset of symptoms, dissolve entire contents of 1 tube in the mouth every 6 hours, up to 3 times per day.
- Children under 2 years of age: Ask a doctor.
do not use if glued carton end flaps are open or if the tray seal is broken
each 0.04 oz dose (1g) contains 1g of sugar
0.04 oz (1 g) each
Meltaway Pellets
Body Aches, Headache, Fever, Chills, Fatigue*
Flu-like Symptoms*
Non-Drowsy No Known Drug Interactions
Works Naturally with your Body
3 Dose
6 Dose
12 Dose
24 Dose
30 Dose
36 Dose
42 Dose
*THESE “USES” HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.