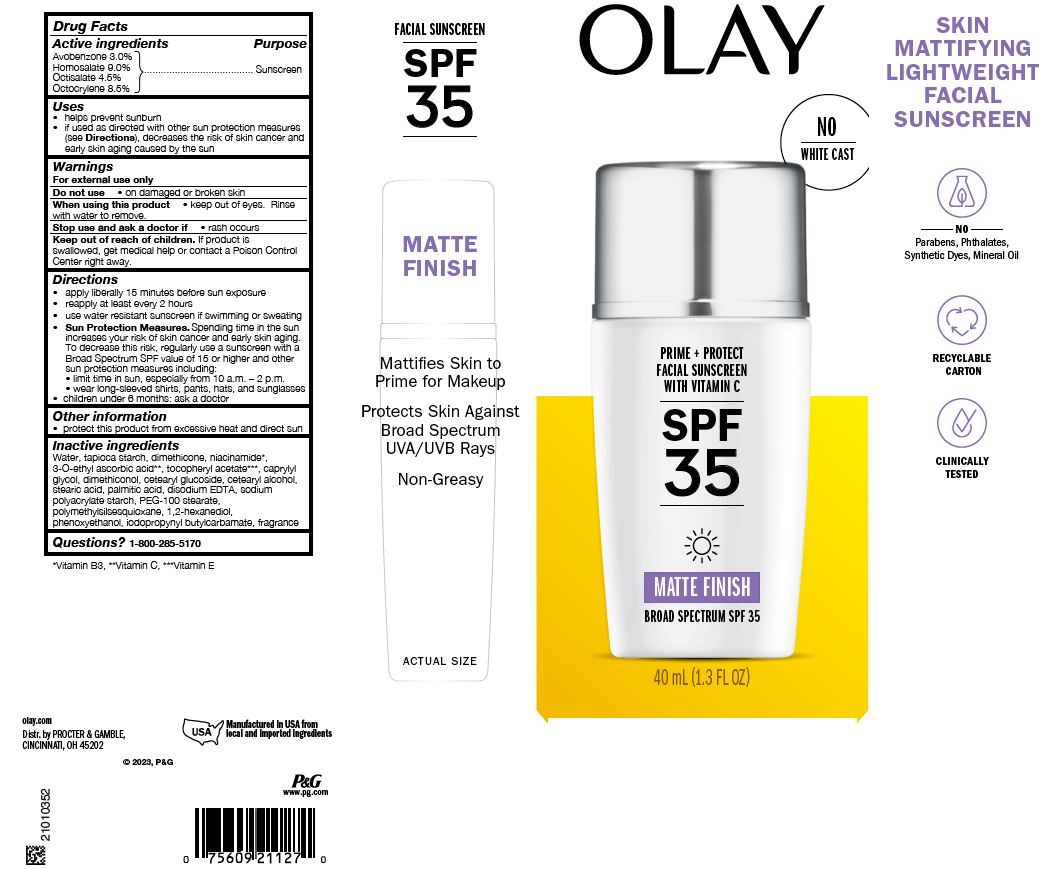
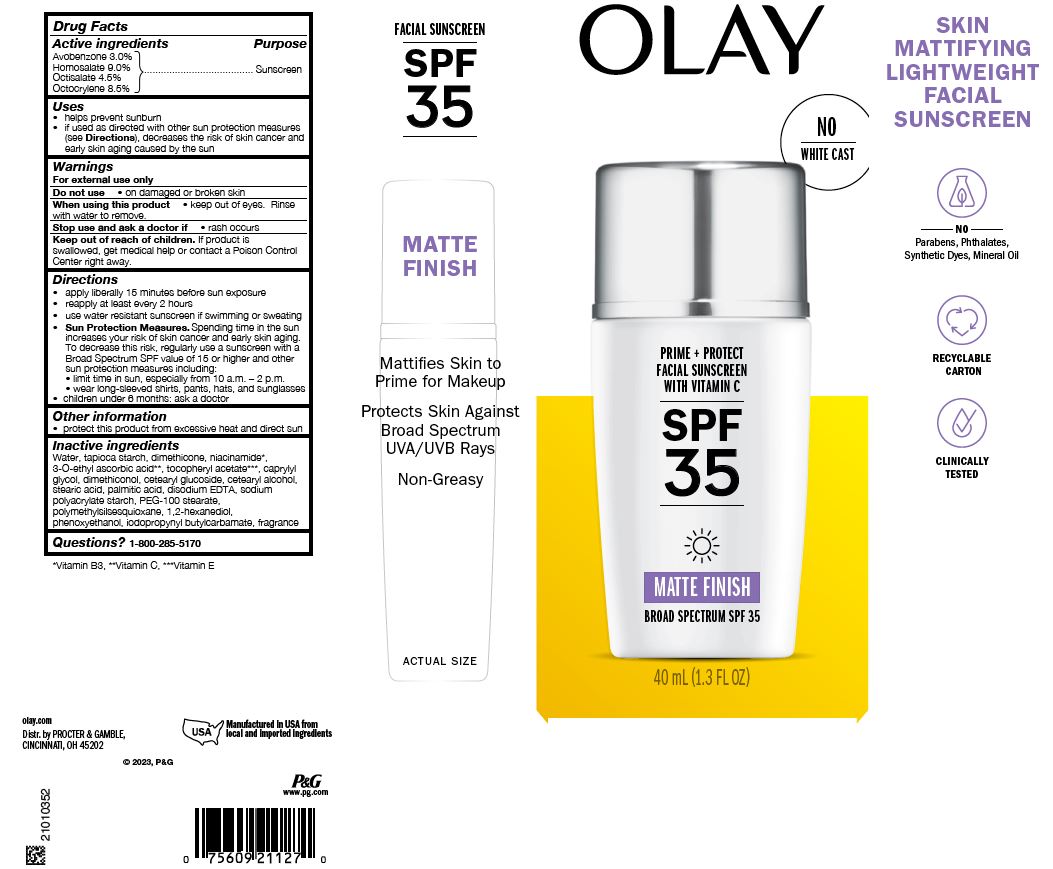
Label: OLAY PRIME PLUS PROTECT FACIAL SUNSCREEN WITH VITAMIN C SPF35 MATTE FINISH- avobenzone, homosalate, octisalate, and octocrylene lotion
- NDC Code(s): 69423-742-40
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months: ask a doctor
- Other information
-
Inactive ingredients
Water, tapioca starch, dimethicone, niacinamide*, 3-O-ethyl ascorbic acid**, tocopheryl acetate***, caprylyl glycol, dimethiconol, cetearyl glucoside, cetearyl alcohol, stearic acid, palmitic acid, disodium EDTA, sodium polyacrylate starch, PEG-100 stearate, polymethylsilsesquioxane, 1,2-hexanediol, phenoxyethanol, iodopropynyl butylcarbamate, fragrance
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1.3 fl oz Carton
-
INGREDIENTS AND APPEARANCE
OLAY PRIME PLUS PROTECT FACIAL SUNSCREEN WITH VITAMIN C SPF35 MATTE FINISH
avobenzone, homosalate, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69423-742 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8.5 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHICONOL (50000 CST) (UNII: R2285D73YT) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) NIACINAMIDE (UNII: 25X51I8RD4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) STEARIC ACID (UNII: 4ELV7Z65AP) EDETATE DISODIUM (UNII: 7FLD91C86K) PEG-100 STEARATE (UNII: YD01N1999R) PALMITIC ACID (UNII: 2V16EO95H1) STARCH, TAPIOCA (UNII: 24SC3U704I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69423-742-40 1 in 1 CARTON 01/15/2024 1 40 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/15/2024 Labeler - The Procter & Gamble Manufacturing Company (004238200)