Label: CHLORPROMAZINE HYDROCHLORIDE tablet, film coated
-
NDC Code(s):
70771-1506-1,
70771-1506-2,
70771-1506-4,
70771-1507-1, view more70771-1507-2, 70771-1507-4, 70771-1508-1, 70771-1508-2, 70771-1508-4, 70771-1509-1, 70771-1509-2, 70771-1509-4, 70771-1510-1, 70771-1510-2, 70771-1510-4
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
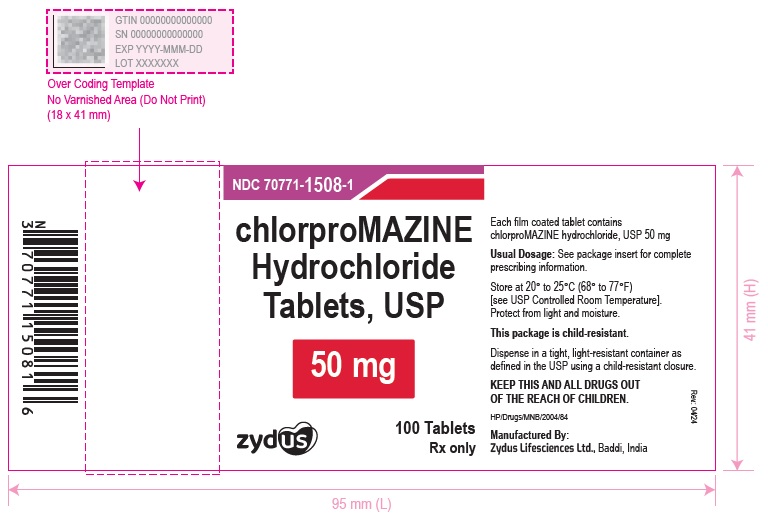
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
chlorproMAZINE Hydrochloride Tablets, USP
10 mg
100 Tablets
Rx only

chlorproMAZINE Hydrochloride Tablets, USP
25 mg
100 Tablets
Rx only

chlorproMAZINE Hydrochloride Tablets, USP
50 mg
100 Tablets
Rx only

chlorproMAZINE Hydrochloride Tablets, USP
100 mg
100 Tablets
Rx only

chlorproMAZINE Hydrochloride Tablets, USP
200 mg
100 Tablets
Rx only

-
INGREDIENTS AND APPEARANCE
CHLORPROMAZINE HYDROCHLORIDE
chlorpromazine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1506 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPROMAZINE HYDROCHLORIDE (UNII: 9WP59609J6) (CHLORPROMAZINE - UNII:U42B7VYA4P) CHLORPROMAZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape ROUND Size 6mm Flavor Imprint Code 11;29 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1506-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/27/2020 2 NDC:70771-1506-4 10 in 1 CARTON 01/27/2020 2 NDC:70771-1506-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213368 01/27/2020 CHLORPROMAZINE HYDROCHLORIDE
chlorpromazine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1507 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPROMAZINE HYDROCHLORIDE (UNII: 9WP59609J6) (CHLORPROMAZINE - UNII:U42B7VYA4P) CHLORPROMAZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape ROUND Size 8mm Flavor Imprint Code 11;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1507-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/27/2020 2 NDC:70771-1507-4 10 in 1 CARTON 01/27/2020 2 NDC:70771-1507-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213368 01/27/2020 CHLORPROMAZINE HYDROCHLORIDE
chlorpromazine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1508 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPROMAZINE HYDROCHLORIDE (UNII: 9WP59609J6) (CHLORPROMAZINE - UNII:U42B7VYA4P) CHLORPROMAZINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape ROUND Size 7mm Flavor Imprint Code 11;31 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1508-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/27/2020 2 NDC:70771-1508-4 10 in 1 CARTON 01/27/2020 2 NDC:70771-1508-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213368 01/27/2020 CHLORPROMAZINE HYDROCHLORIDE
chlorpromazine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1509 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPROMAZINE HYDROCHLORIDE (UNII: 9WP59609J6) (CHLORPROMAZINE - UNII:U42B7VYA4P) CHLORPROMAZINE HYDROCHLORIDE 100 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape ROUND Size 10mm Flavor Imprint Code 11;32 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1509-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/27/2020 2 NDC:70771-1509-4 10 in 1 CARTON 01/27/2020 2 NDC:70771-1509-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213368 01/27/2020 CHLORPROMAZINE HYDROCHLORIDE
chlorpromazine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1510 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPROMAZINE HYDROCHLORIDE (UNII: 9WP59609J6) (CHLORPROMAZINE - UNII:U42B7VYA4P) CHLORPROMAZINE HYDROCHLORIDE 200 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape ROUND Size 12mm Flavor Imprint Code 11;33 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1510-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/27/2020 2 NDC:70771-1510-4 10 in 1 CARTON 01/27/2020 2 NDC:70771-1510-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213368 01/27/2020 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (677605858) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 677605858 ANALYSIS(70771-1506, 70771-1507, 70771-1508, 70771-1509, 70771-1510) , MANUFACTURE(70771-1506, 70771-1507, 70771-1508, 70771-1509, 70771-1510)