PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
ChlorproMAZINE Hydrochloride Tablets, USP
10 mg
100 Tablets
Rx only

ChlorproMAZINE Hydrochloride Tablets, USP
25 mg
100 Tablets
Rx only

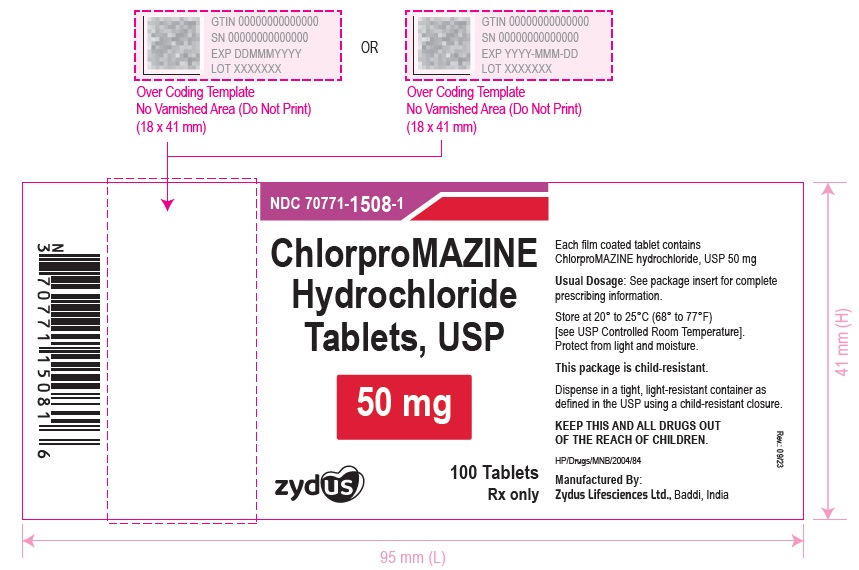
ChlorproMAZINE Hydrochloride Tablets, USP
50 mg
100 Tablets
Rx only
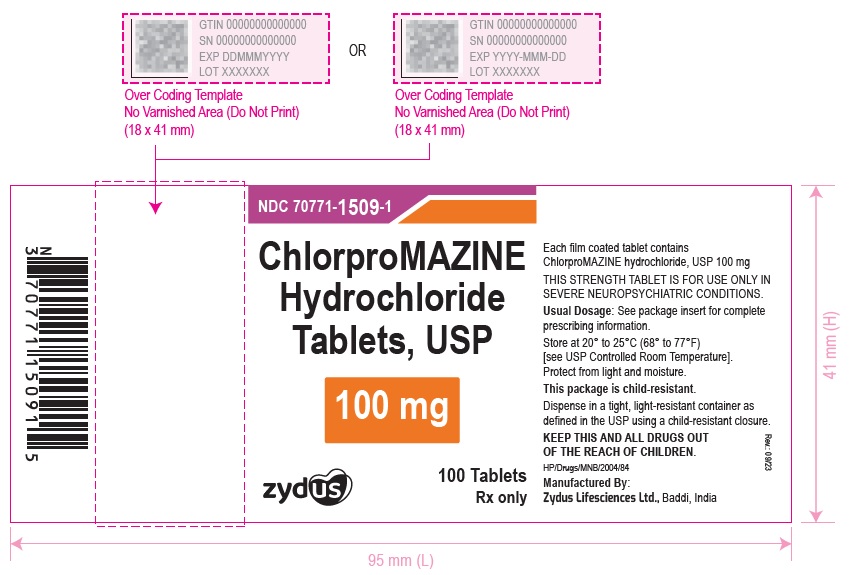
ChlorproMAZINE Hydrochloride Tablets, USP
100 mg
100 Tablets
Rx only
ChlorproMAZINE Hydrochloride Tablets, USP
200 mg
100 Tablets
Rx only
