Label: CHOICE HAND SANITIZE- ethyl alcohol gel
- NDC Code(s): 58575-183-01, 58575-183-02
- Packager: Inopak. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Keep out of reach of children
- Usage
- Warnings
- Other Information
- Stop Usage
- Administration
- Inactive Ingredients
-
Choice Box Container
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v ……………Antiseptic
Uses
- To decrease bacteria on the skin that potentially can cause disease
- Recommended for repeated use
Warnings
- Flammable, keep away from heat or flame.
- For external use only.
Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water.
Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours.
Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions
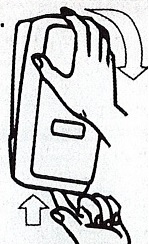
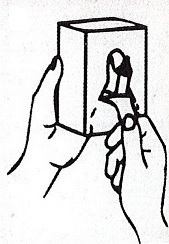
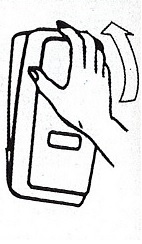
- Apply sufficient amount of product to your palm to cover both hands.
- Rub until dry.
Other Information
May discolor certain fabrics and surfaces.
Inactive Ingredients
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
Choice
HAND SANITIZING GEL
Aloe Ver
And
Vitamin E
Instant
Waterless
Contains
Moisturizers
PN 5025-404UV-CH
800mHand sanitizing gel
- Contains Aloe Vera and Vitamin E
- Instant waterless with moisturizers
- Produced in an FDA registered facility
- cGMP facility
- Dries quickly leaving the skin feeling soothed and moisturized
Kills
99.9% of
Germs
MADE IN THE USAChoice
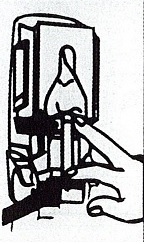
Dispenser Loading Instructions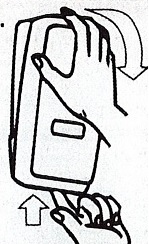
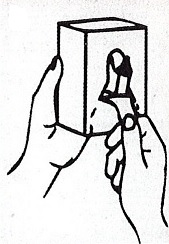
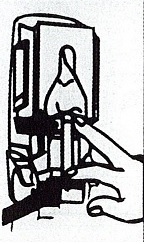
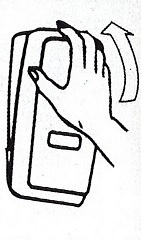
Section 1 Section 2 Section 3 Section 4

- Choice Case
-
INGREDIENTS AND APPEARANCE
CHOICE HAND SANITIZE
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58575-183 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ISOPROPYL ALCOHOL (UNII: ND2M416302) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58575-183-01 800 mL in 1 POUCH; Type 0: Not a Combination Product 01/02/2020 07/11/2024 2 NDC:58575-183-02 9600 mL in 1 CASE; Type 0: Not a Combination Product 01/02/2020 07/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/02/2020 07/11/2024 Labeler - Inopak. Ltd (194718243) Establishment Name Address ID/FEI Business Operations Inopak. Ltd 194718243 manufacture(58575-183)