Keep out of reach of children
Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Usage
Uses
- To decrease bacteria on the skin that potentially can cause disease
- Recommended for repeated use
Other Information
Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water
May discolor certain fabrics and surfaces.
Stop Usage
Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours.
Administration
Directions
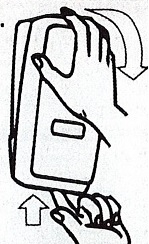
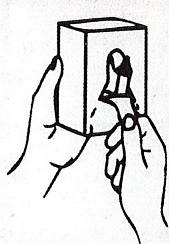
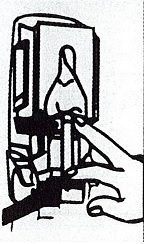
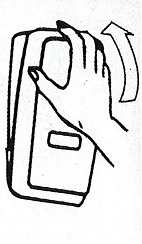
- Apply sufficient amount of product to your palm to cover both hands.
- Rub until dry.
Inactive Ingredients
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
Choice Box Container
|
Drug Facts Active Ingredient Purpose Ethyl Alcohol 70% v/v ……………Antiseptic Uses
Warnings
Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product. Directions
Other Information May discolor certain fabrics and surfaces. Inactive Ingredients Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water |
Choice HAND SANITIZING GEL Aloe Ver And Vitamin E Instant Waterless Contains Moisturizers PN 5025-404UV-CH 800m |
Hand sanitizing gel
Kills 99.9% of Germs MADE IN THE USA |
Choice Dispenser Loading Instructions |
Section 1 Section 2 Section 3 Section 4

