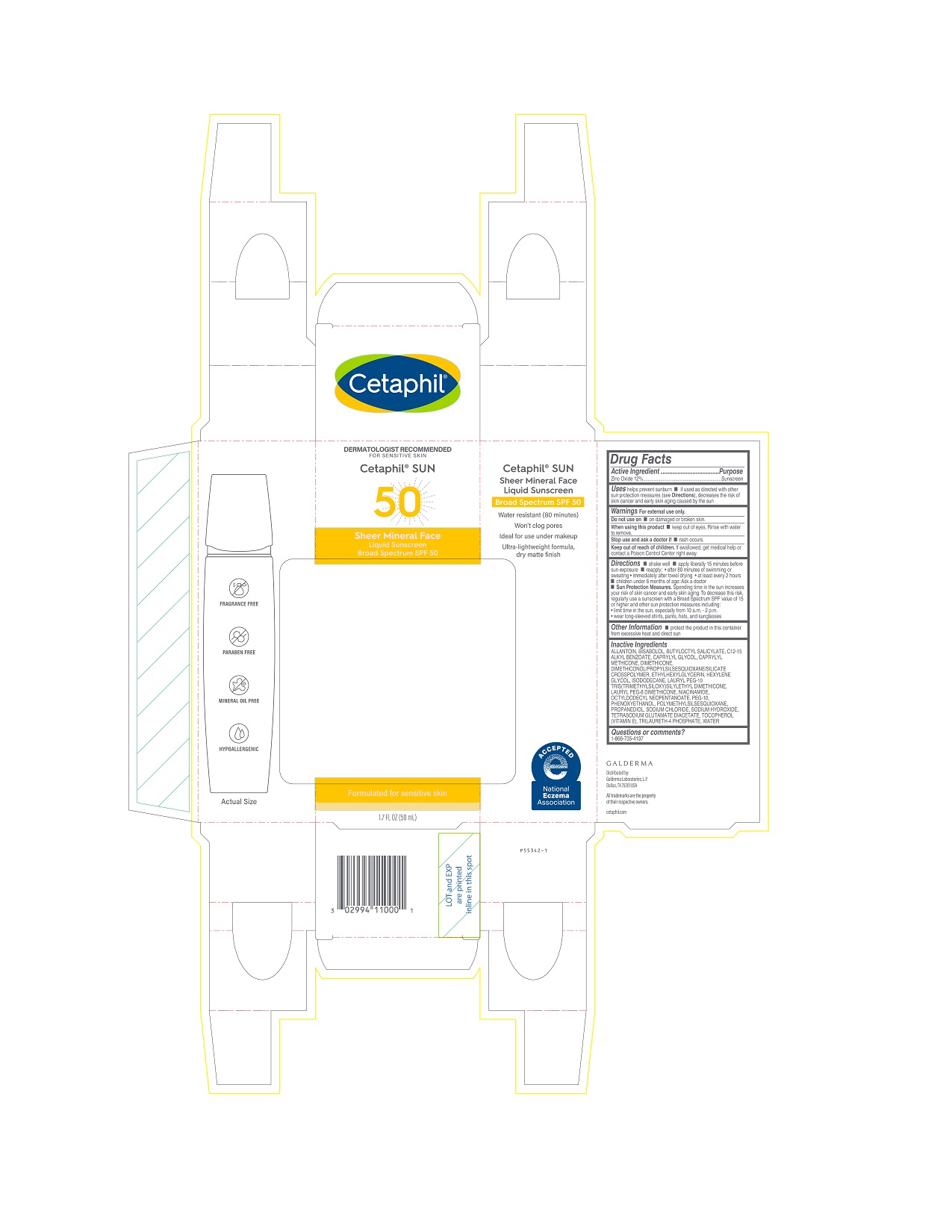
Label: CETAPHIL SHEER MINERAL FACE LIQUID SUNSCREEN SPF 50- zinc oxide lotion
- NDC Code(s): 0299-4110-00, 0299-4110-05
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- PURPOSE
- Uses
- Warnings For external use only
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- shake well
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Other Information
▪ protect the product in this container from excessive heat and direct sun
-
Inactive Ingredients
ALLANTOIN, BISABOLOL, BUTYLOCTYL SALICYLATE, C12-15 ALKYL BENZOATE, CAPRYLYL GLYCOL, CAPRYLYL METHICONE, DIMETHICONE, DIMETHICONOL/PROPYLSILSESQUIOXANE/SILICATE CROSSPOLYMER, ETHYLHEXYLGLYCERIN, HEXYLENE GLYCOL, ISODODECANE, LAURYL PEG-10 TRIS(TRIMETHYLSILOXY)SILYLETHYL DIMETHICONE, LAURYL PEG-8 DIMETHICONE, NIACINAMIDE, OCTYLDODECYL NEOPENTANOATE, PEG-10, PHENOXYETHANOL, POLYMETHYLSILSESQUIOXANE, PROPANEDIOL, SODIUM CHLORIDE,SODIUM HYDROXIDE, TETRASODIUM GLUTAMATE DIACETATE, TOCOPHEROL (VITAMIN E), TRILAURETH-4 PHOSPHATE, WATER
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 1.7 FL OZ CARTON
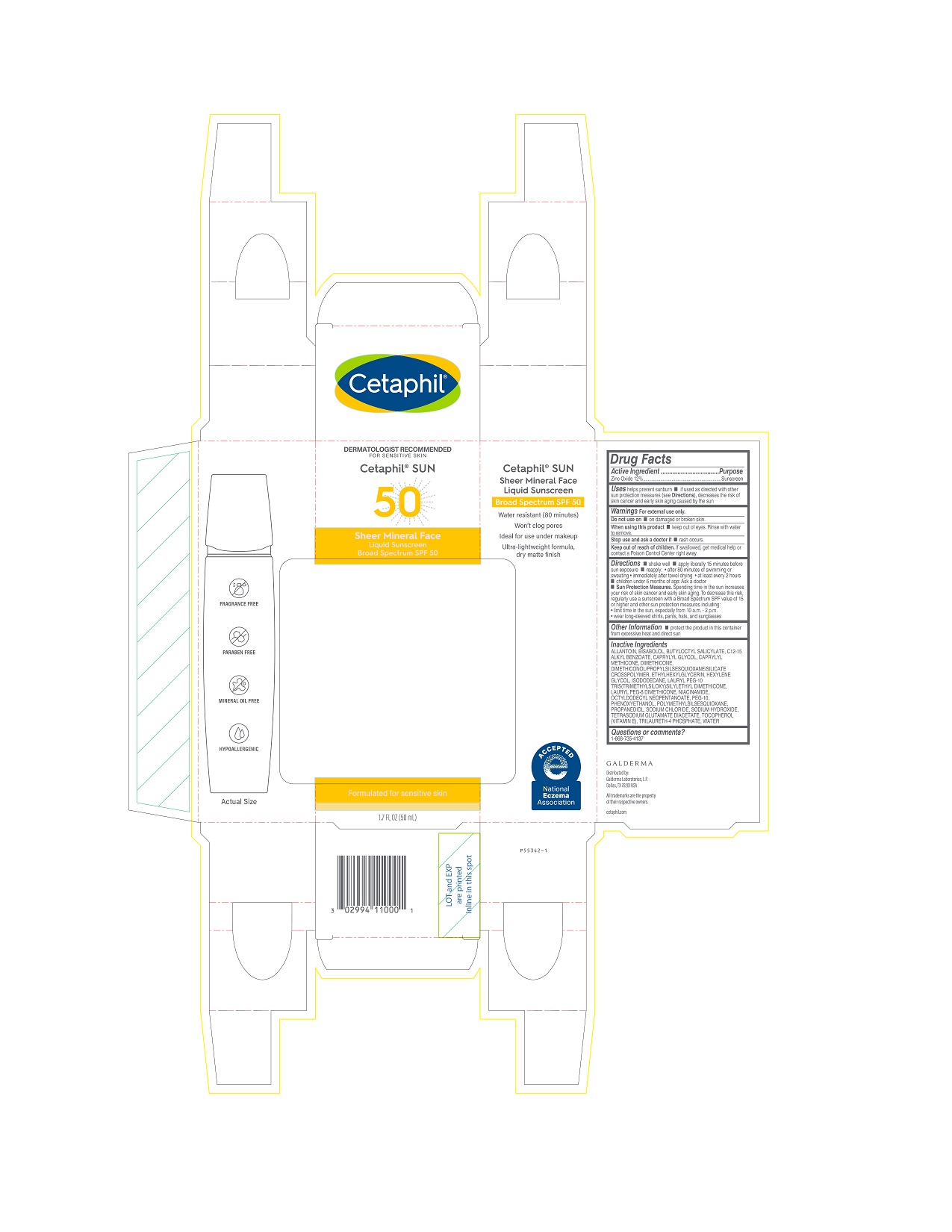
DERMATOLOGIST RECOMMENDED
FOR SENSITIVE SKIN
Cetaphil® SUN
50
Sheer Mineral Face
Liquid SunscreenBroad Spectrum SPF 50
Formulated for sensitive skin
1.7 FL OZ (50 mL)
Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
cetaphil.com
P55342-1
PRINCIPAL DISPLAY PANEL - 1.7 FL OZ FRONT LABEL

Cetaphil®
SUN
50
Sheer Mineral Face
Liquid Sunscreen
Broad Spectrum SPF 50
Formulated for sensitive skin
Microbiome gentle
Water resistant (80 minutes)
Ideal for use under makeup
Vitamin E
1.7 FL OZ (50mL)
P55341-1
-
INGREDIENTS AND APPEARANCE
CETAPHIL SHEER MINERAL FACE LIQUID SUNSCREEN SPF 50
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength Allantoin (UNII: 344S277G0Z) Levomenol (UNII: 24WE03BX2T) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Caprylyl Glycol (UNII: 00YIU5438U) Caprylyl Trisiloxane (UNII: Q95M2P1KJL) Dimethicone (UNII: 92RU3N3Y1O) Dimethiconol/Propylsilsesquioxane/Silicate Crosspolymer (450000000 Mw) (UNII: 9KB5R958PB) Ethylhexylglycerin (UNII: 147D247K3P) Hexylene Glycol (UNII: KEH0A3F75J) Isododecane (UNII: A8289P68Y2) Niacinamide (UNII: 25X51I8RD4) Octyldodecyl Neopentanoate (UNII: X8725R883T) Polyethylene Glycol 500 (UNII: 761NX2Q08Y) Phenoxyethanol (UNII: HIE492ZZ3T) Polymethylsilsesquioxane (4.5 Microns) (UNII: 59Z907ZB69) Propanediol (UNII: 5965N8W85T) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Tetrasodium Glutamate Diacetate (UNII: 5EHL50I4MY) Tocopherol (UNII: R0ZB2556P8) Trilaureth-4 Phosphate (UNII: M96W2OLL2V) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4110-00 1 in 1 CARTON 11/01/2020 1 50 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:0299-4110-05 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2020 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 623502044 manufacture(0299-4110)