Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Other Information
▪ protect the product in this container from excessive heat and direct sun
Inactive Ingredients
ALLANTOIN, BISABOLOL, BUTYLOCTYL SALICYLATE, C12-15 ALKYL BENZOATE, CAPRYLYL GLYCOL, CAPRYLYL METHICONE, DIMETHICONE, DIMETHICONOL/PROPYLSILSESQUIOXANE/SILICATE CROSSPOLYMER, ETHYLHEXYLGLYCERIN, HEXYLENE GLYCOL, ISODODECANE, LAURYL PEG-10 TRIS(TRIMETHYLSILOXY)SILYLETHYL DIMETHICONE, LAURYL PEG-8 DIMETHICONE, NIACINAMIDE, OCTYLDODECYL NEOPENTANOATE, PEG-10, PHENOXYETHANOL, POLYMETHYLSILSESQUIOXANE, PROPANEDIOL, SODIUM CHLORIDE,SODIUM HYDROXIDE, TETRASODIUM GLUTAMATE DIACETATE, TOCOPHEROL (VITAMIN E), TRILAURETH-4 PHOSPHATE, WATER
PRINCIPAL DISPLAY PANEL - 1.7 FL OZ CARTON
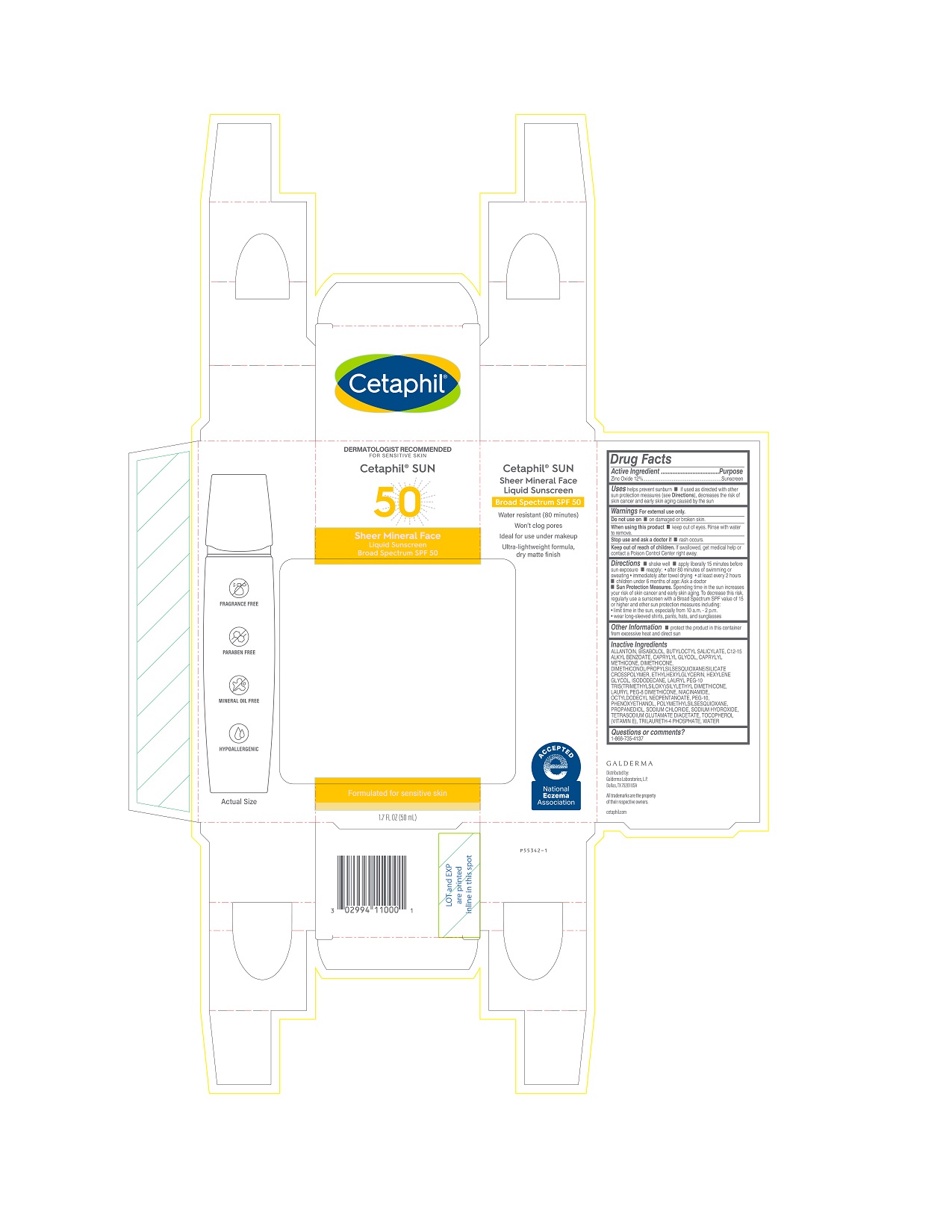
DERMATOLOGIST RECOMMENDED
FOR SENSITIVE SKIN
Cetaphil® SUN
50
Sheer Mineral Face
Liquid Sunscreen
Broad Spectrum SPF 50
Formulated for sensitive skin
1.7 FL OZ (50 mL)
Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
cetaphil.com
P55342-1
PRINCIPAL DISPLAY PANEL - 1.7 FL OZ FRONT LABEL

Cetaphil®
SUN
50
Sheer Mineral Face
Liquid Sunscreen
Broad Spectrum SPF 50
Formulated for sensitive skin
Microbiome gentle
Water resistant (80 minutes)
Ideal for use under makeup
Vitamin E
1.7 FL OZ (50mL)
P55341-1