Label: BEDSIDE-CARE- benzethonium chloride shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 11701-020-04, 11701-020-05, 11701-020-09 - Packager: Coloplast Manufacturing US, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 30, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
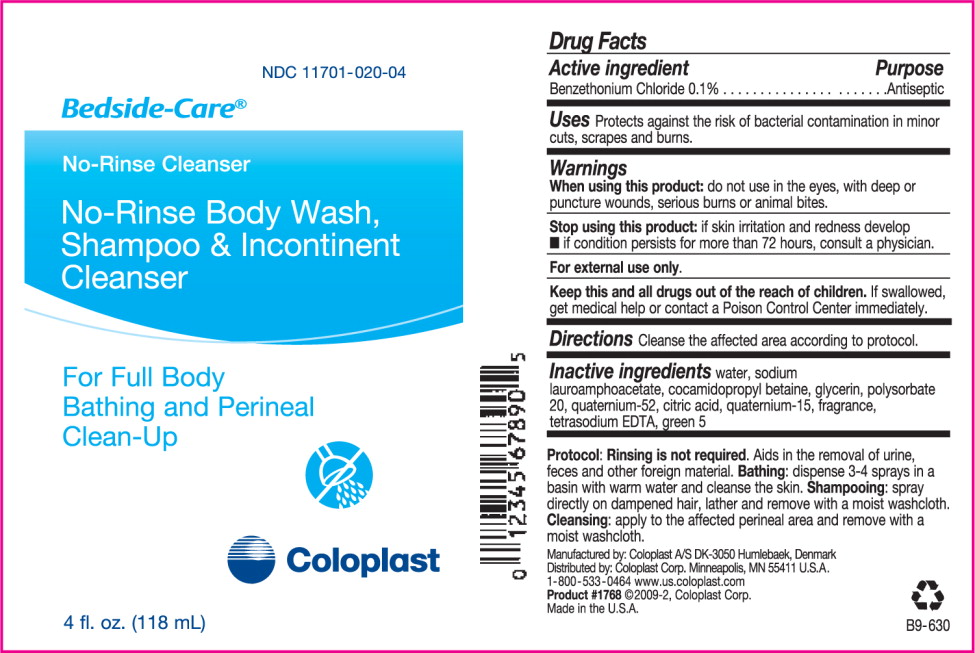
Drug Facts
When using this product: do not use in the eyes, with deep or puncture wounds, serious burns or animal bites.
Stop using this product:
- if skin irritation and redness develop
- if condition persists for more than 72 hours, consult a physician.
Keep this and all drugs out of the reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Inactive ingredients water, sodium lauroamphoacetate, cocamidopropyl betaine, glycerin, polysorbate 20, quaternium-52, citric acid, quaternium-15, fragrance, tetrasodium EDTA, green 5
Protocol: Rinsing is not required. Aids in the removal of urine, feces and other foreign material. Bathing: dispense 3-4 sprays in a basin with warm water and cleanse the skin. Shampooing: spray directly on dampened hair, lather and remove with a mois washcloth. Cleansing: apply to the affected perineal area and remove with a moist washcloth.
Manufactured by: Coloplast A/S
DK-3050 Humlebaek, Denmark
Distributed by: Coloplast Corp.
Minneapolis, MN 55411 U.S.A.
1-800-533-0464 www.us.coloplast.com
Product #1762 ©2009-2, Coloplast Corp.
Made in the U.S.A.B9-612
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BEDSIDE-CARE
benzethonium chloride shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11701-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 1 mL in 1000 mL Inactive Ingredients Ingredient Name Strength D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) CITRIC ACID (UNII: 2968PHW8QP) EDETATE SODIUM (UNII: MP1J8420LU) QUATERNIUM-15 (UNII: E40U03LEM0) POLYSORBATE 20 (UNII: 7T1F30V5YH) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) QUATERNIUM-52 (UNII: 588EQF3H1P) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11701-020-04 118 mL in 1 BOTTLE, SPRAY 2 NDC:11701-020-05 237 mL in 1 BOTTLE, SPRAY 3 NDC:11701-020-09 3800 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 06/15/2009 Labeler - Coloplast Manufacturing US, LLC (110326675) Registrant - Coloplast Corp (847436391) Establishment Name Address ID/FEI Business Operations Coloplast Manufacturing US, LLC 110326675 MANUFACTURE