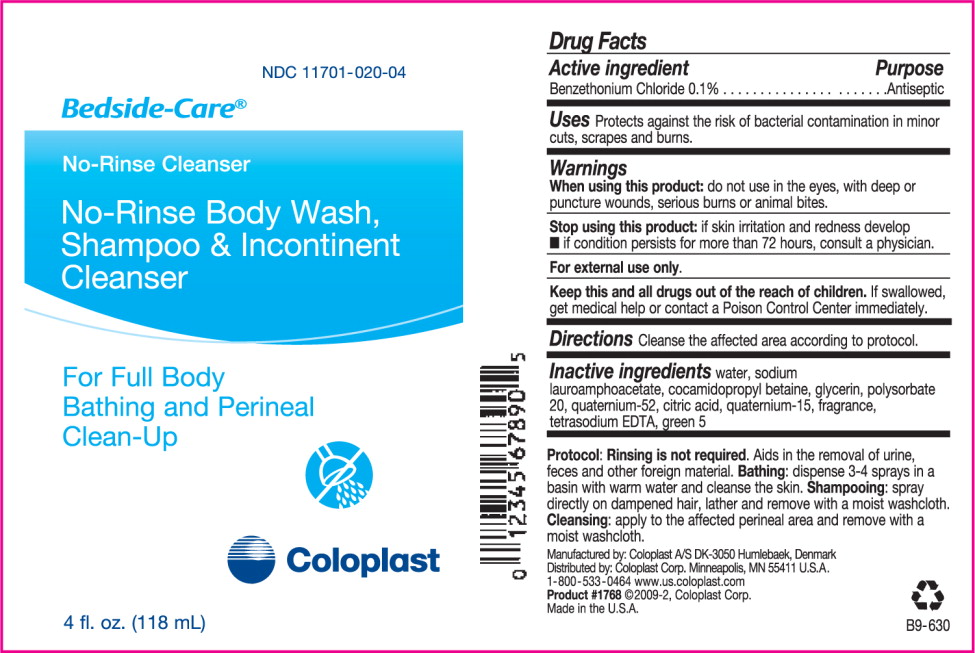
Drug Facts
When using this product: do not use in the eyes, with deep or puncture wounds, serious burns or animal bites.
Stop using this product:
- if skin irritation and redness develop
- if condition persists for more than 72 hours, consult a physician.
Keep this and all drugs out of the reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Inactive ingredients water, sodium lauroamphoacetate, cocamidopropyl betaine, glycerin, polysorbate 20, quaternium-52, citric acid, quaternium-15, fragrance, tetrasodium EDTA, green 5
Protocol: Rinsing is not required. Aids in the removal of urine, feces and other foreign material. Bathing: dispense 3-4 sprays in a basin with warm water and cleanse the skin. Shampooing: spray directly on dampened hair, lather and remove with a mois washcloth. Cleansing: apply to the affected perineal area and remove with a moist washcloth.
Manufactured by: Coloplast A/S
DK-3050 Humlebaek, Denmark
Distributed by: Coloplast Corp.
Minneapolis, MN 55411 U.S.A.
1-800-533-0464 www.us.coloplast.com
Product #1762 ©2009-2, Coloplast Corp.
Made in the U.S.A.
B9-612