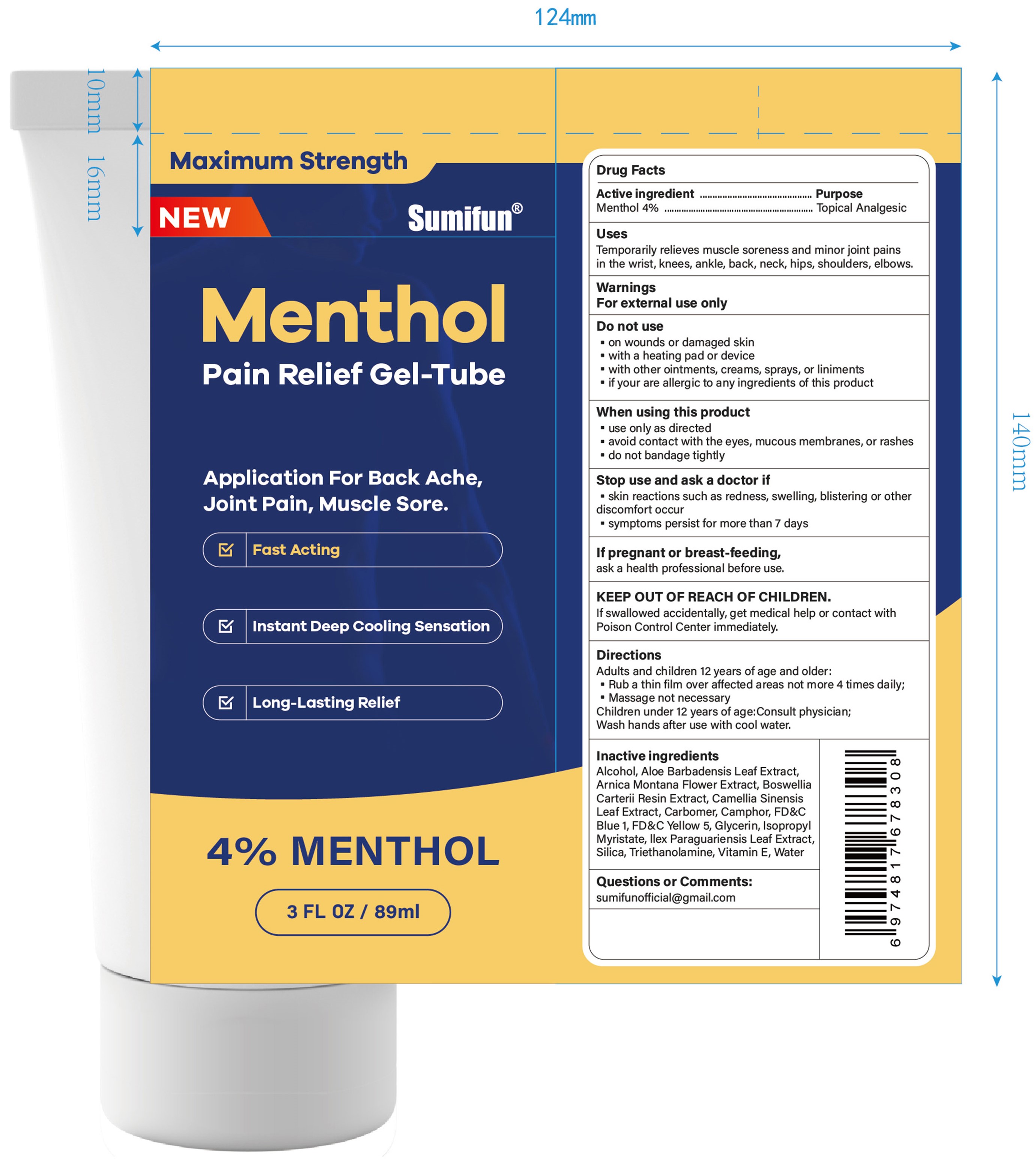
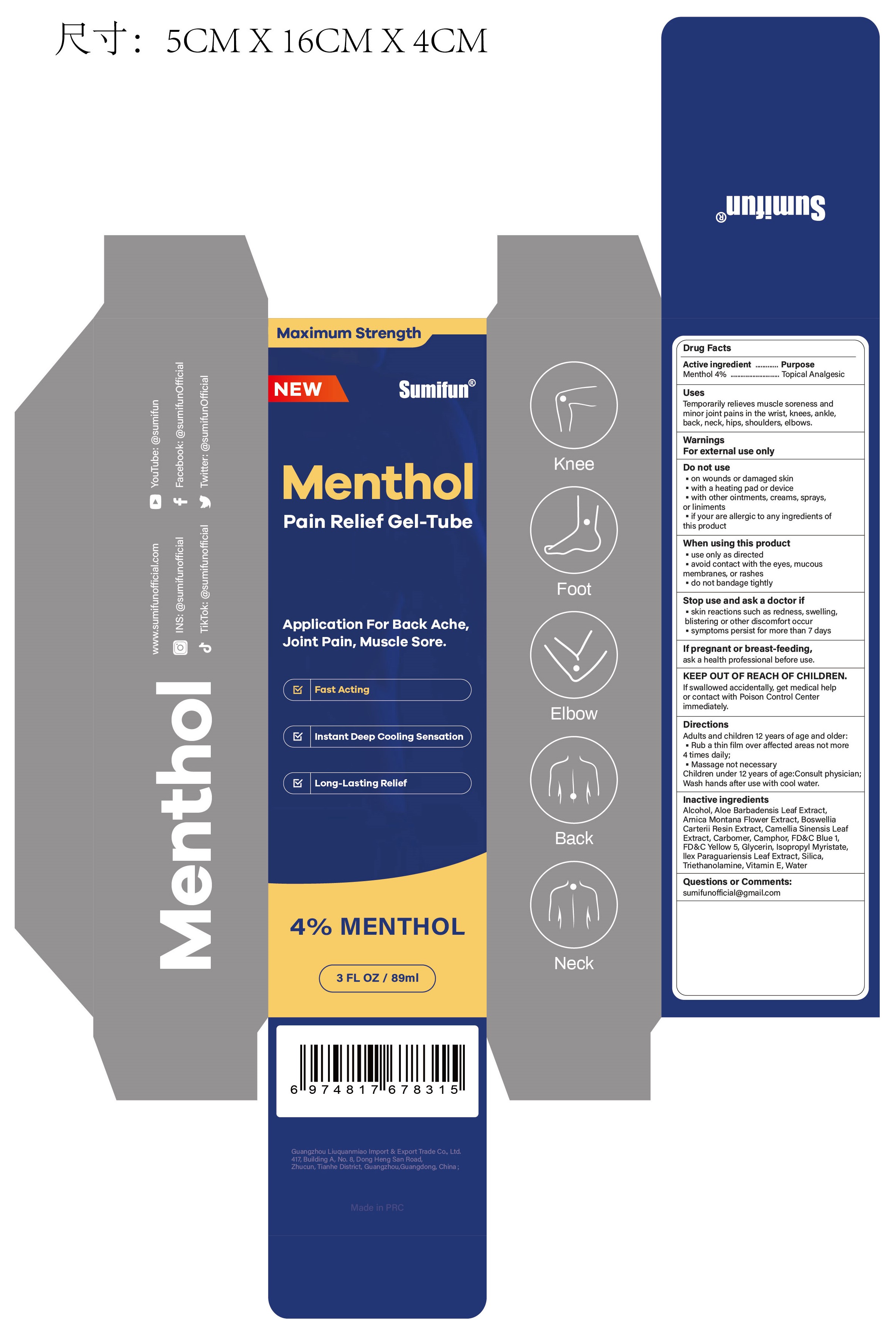
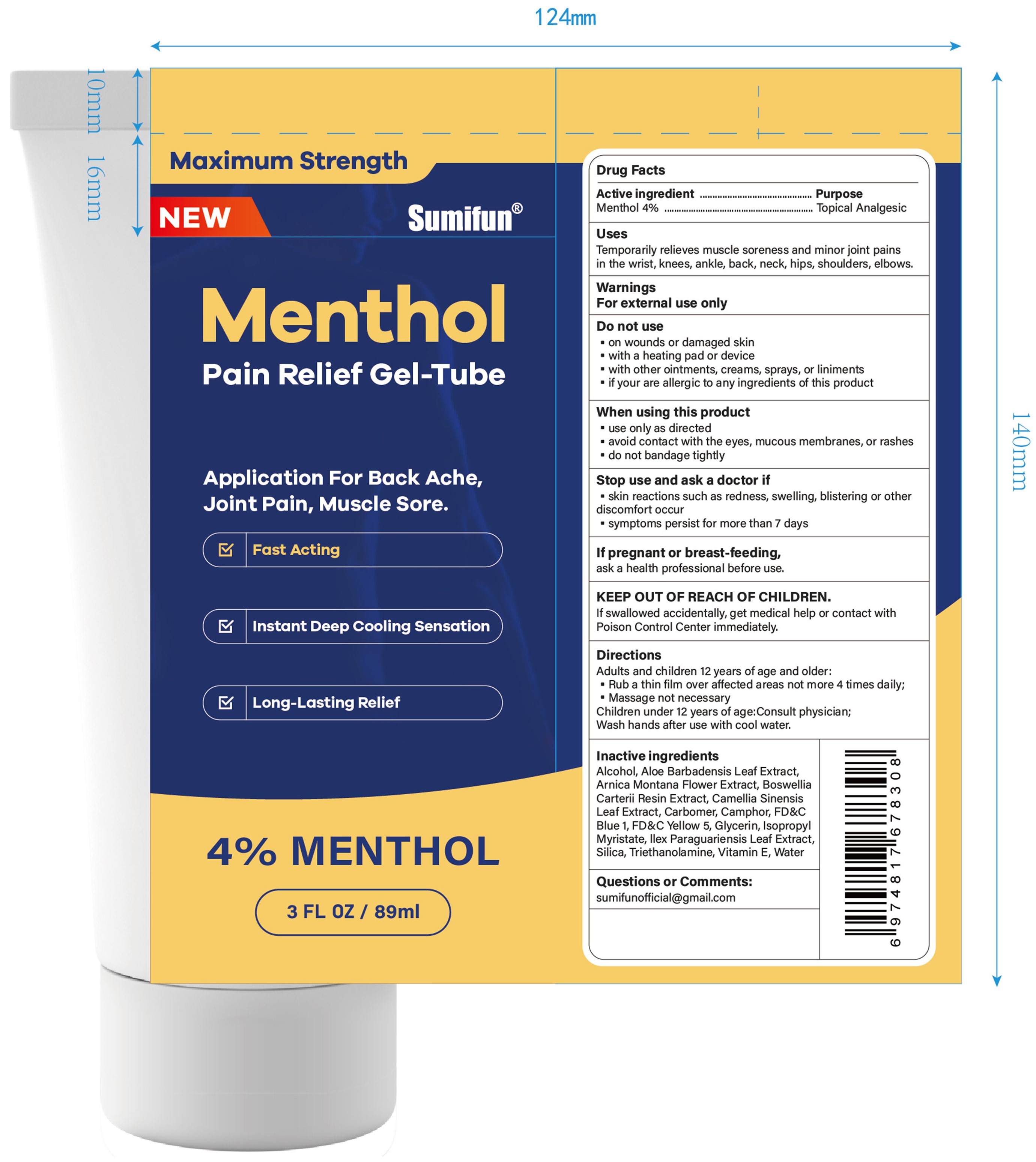
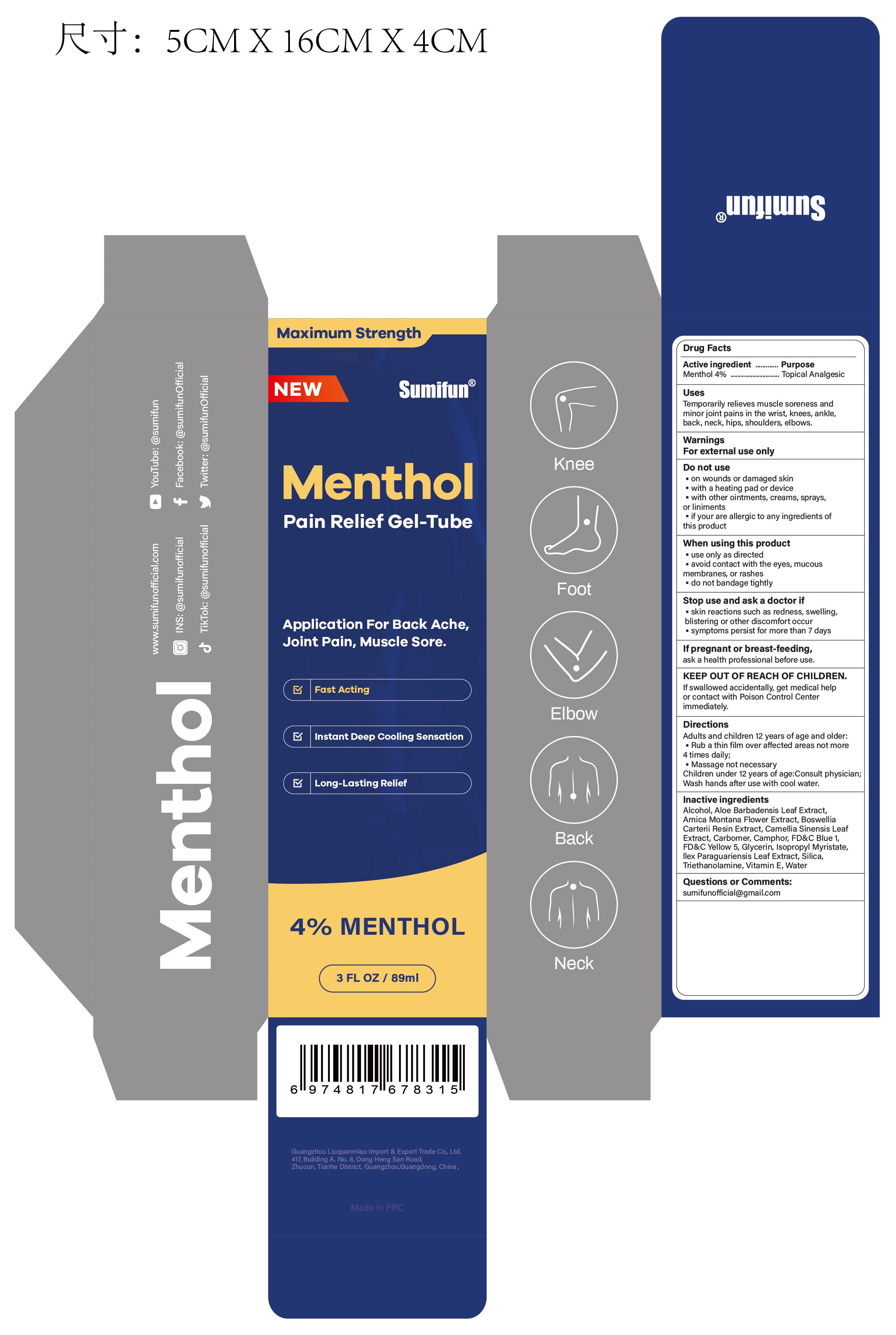
Label: MENTHOL PAIN RELIEF GEL-TUBE- menthol gel
- NDC Code(s): 83602-323-30
- Packager: Guangzhou Liuquanmiao Import & Export Trade Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use ... if
- ... ask a doctor if
- If pregnant or breast-feeding,
- KEEP OUT OF REACH OF CHILDREN.
- Directions
- Inactive ingredients
- Questions or Comments:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MENTHOL PAIN RELIEF GEL-TUBE
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83602-323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.04 g in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL 95% (UNII: 7528N5H79B) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) FRANKINCENSE (UNII: R9XLF1R1WM) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83602-323-30 89 mL in 1 TUBE; Type 0: Not a Combination Product 12/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/06/2023 Labeler - Guangzhou Liuquanmiao Import & Export Trade Co., Ltd. (418399704) Registrant - Guangzhou Liuquanmiao Import & Export Trade Co., Ltd. (418399704) Establishment Name Address ID/FEI Business Operations Shanghai Chuangshi Medical Technology (Group) Co., Ltd. 546872672 manufacture(83602-323) , label(83602-323)