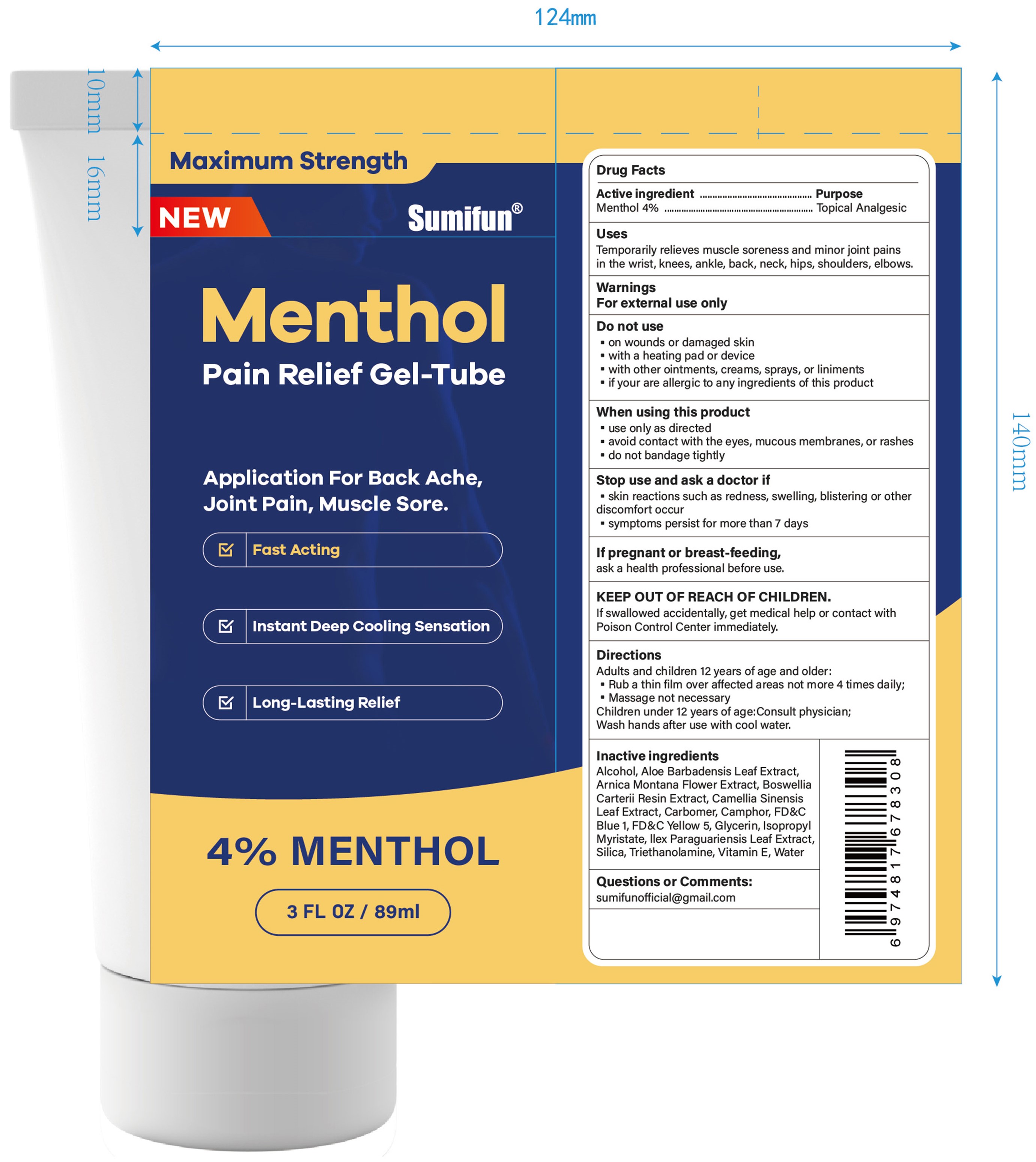
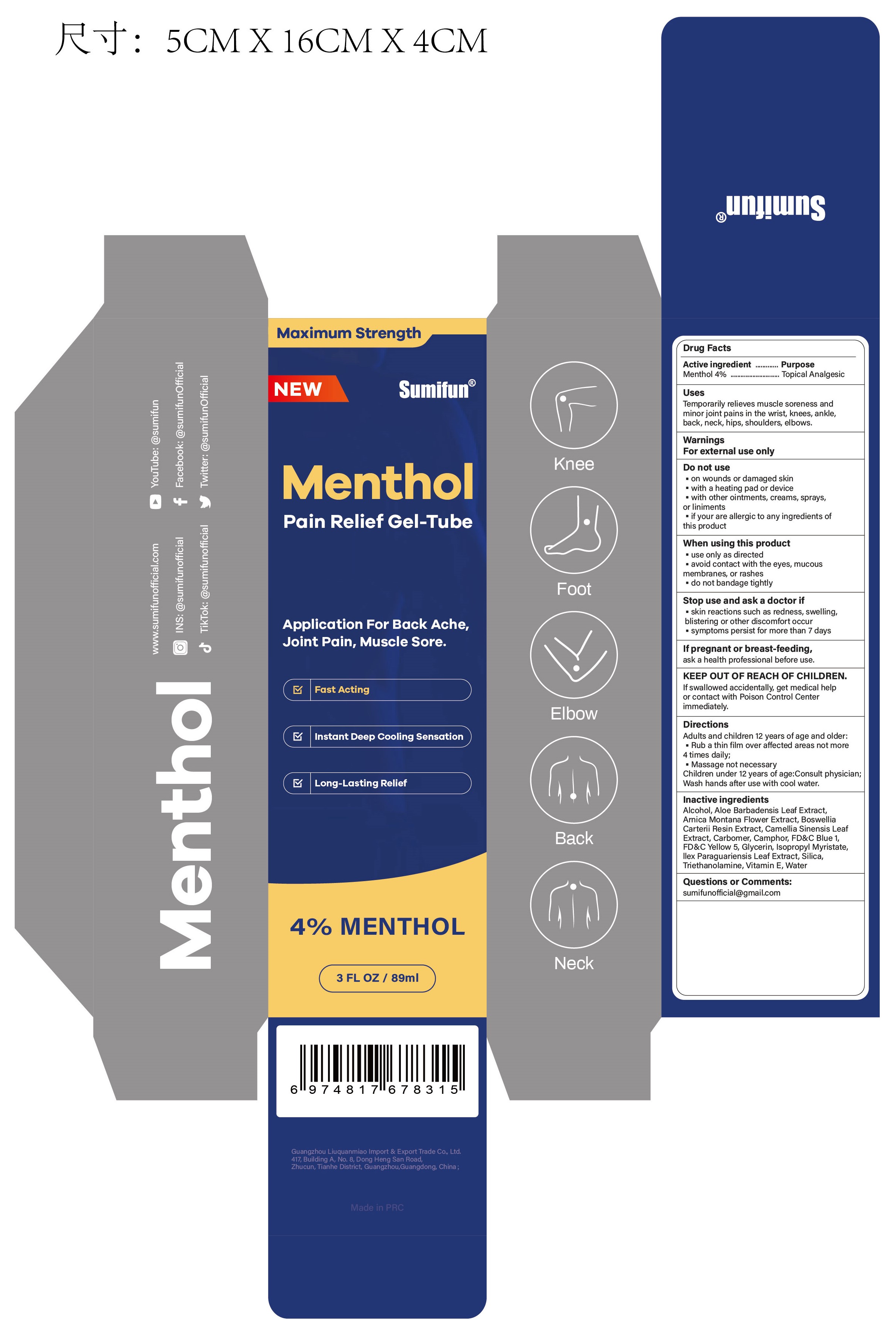
Uses
Temporarily relieves muscle soreness and minor joint pains in the wrist, knees, ankle, back, neck, hips, shoulders, elbows.
Do not use
• on wounds or damaged skin
• with a heating pad or device
• with other ointments, creams, sprays, or liniments
• if you are allergic to any ingredients of this product
When using this product
• use only as directed
• avoid contact with the eyes, mucous membranes, or rashes
• do not bandage tightly
Stop use ... if
• skin reactions such as redness, swelling, blistering or other discomfort occur
• symptoms persist for more than 7 days
... ask a doctor if
• skin reactions such as redness, swelling, blistering or other discomfort occur
• symptoms persist for more than 7 days
KEEP OUT OF REACH OF CHILDREN.
If swallowed accidentally, get medical help or contact with Poison Control Center immediately.
Directions
Adults and children 12 years of age and older:
• Rub a thin film over affected areas not more than 4 times daily;
• Message not necessary
Children under 12 years of age: Consult physician.
Wash hands after use with cool water.
Inactive ingredients
Alcohol, Aloe Barbadensis Leaf Extract, Arnica Montana Flower Extract, Boswellia Carterii Resin Extract, Camellia Sinensis Leaf Extract, Carbomer, Camphor, FD&C Blue 1, FD&C Yellow 5, Glycerin, Isopropyl Myristate, Ilex Paraguariensis Leaf Extract, Silica, Triethanolamine, Vitamin E, Water