Label: COLD AND FLU NIGHTTIME- acetaminophen, dextromethorphan hbr, triprolidine hcl solution
- NDC Code(s): 51316-963-45
- Packager: CVS WOONSOCKET PRESCRIPTION CENTER, INCORPORATED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
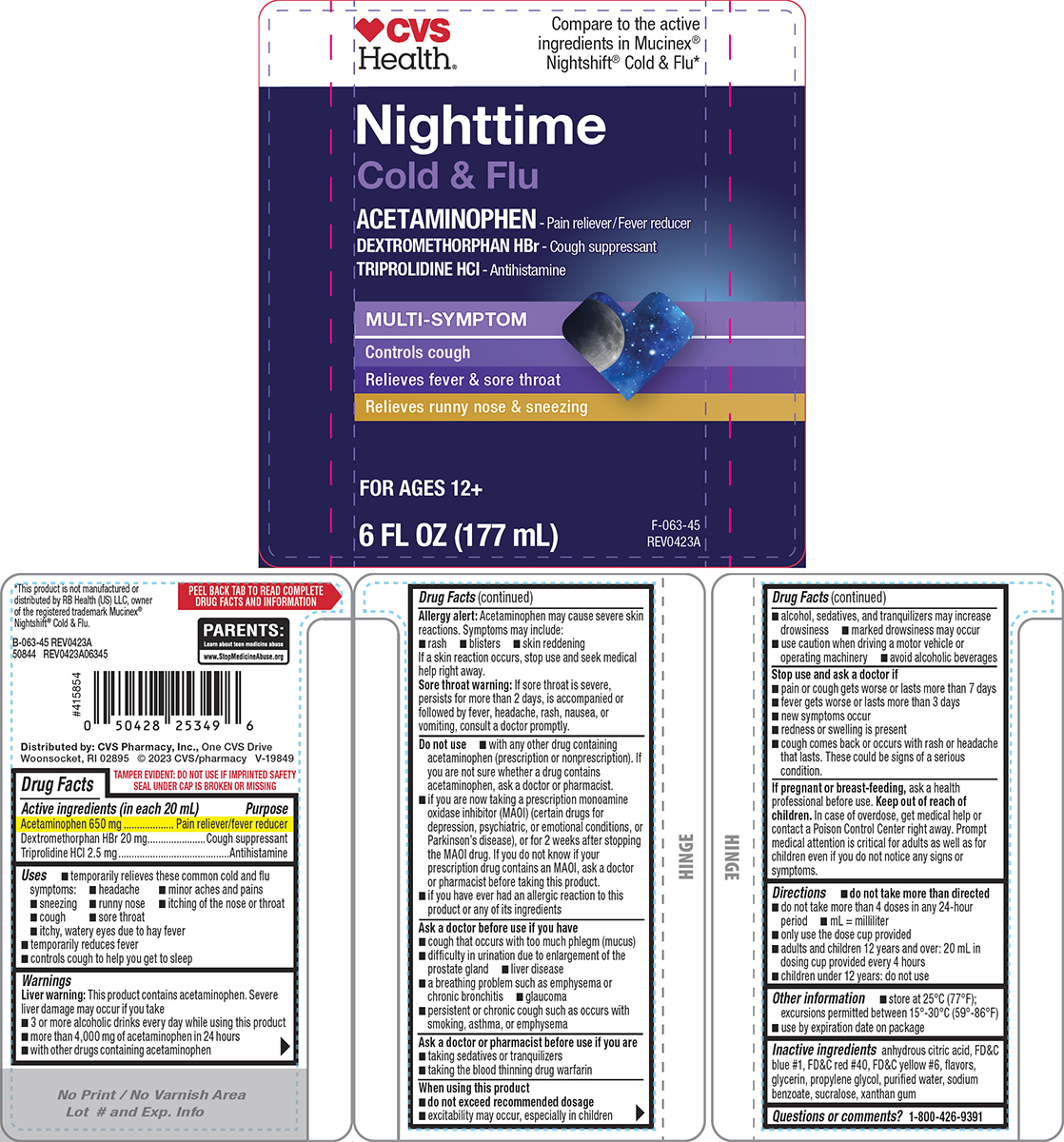
- Active ingredients (in each 20 mL)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- 3 or more alcoholic drinks every day while using this product
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- rash
- blisters
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- alcohol, sedatives, and tranquilizers may increase drowsiness
- marked drowsiness may occur
- use caution when driving a motor vehicle or operating machinery
- avoid alcoholic beverages
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
♥CVS
Health®Compare to the active
ingredients in Mucinex®
Nightshift® Cold & Flu*Nighttime
Cold & Flu
ACETAMINOPHEN - Pain reliever/Fever reducer
DEXTROMETHORPHAN HBr - Cough suppressant
TRIPROLIDINE HCl - AntihistamineMULTI-SYMPTOM
Controls cough
Relieves fever & sore throat
Relieves runny nose & sneezingFOR AGES 12+
6 FL OZ (177 mL)
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSINGPARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.org*This product is not manufactured or
distributed by RB Health (US) LLC, owner
of the registered trademark Mucinex®
Nightshift® Cold & Flu.B-063-45 REV0423A
50844 REV0423A06345Distributed by: CVS Pharmacy, Inc., One CVS Drive
Woonsocket, RI 02895 © 2023 CVS/pharmacy V-19849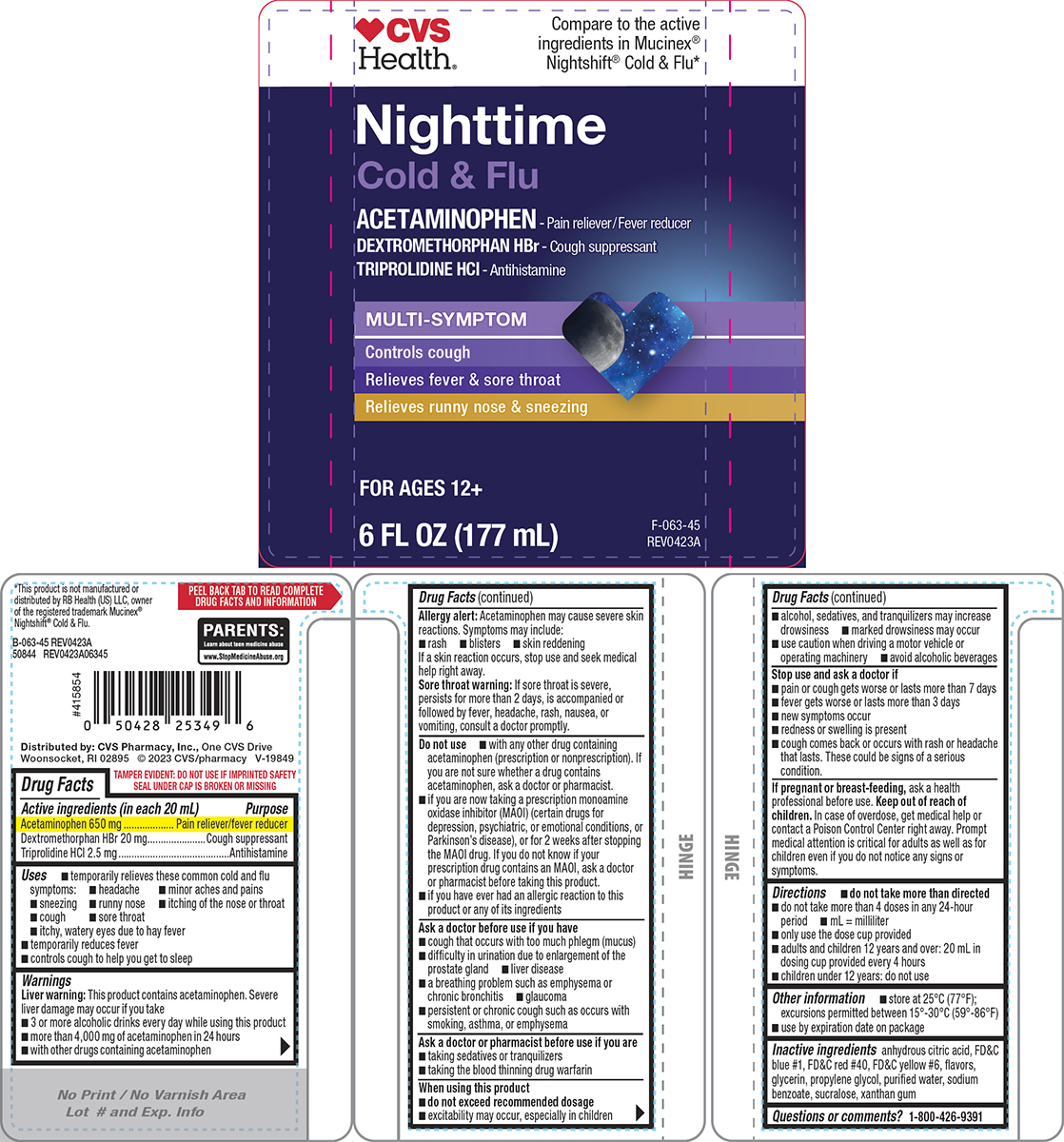
CVS 44-063
-
INGREDIENTS AND APPEARANCE
COLD AND FLU NIGHTTIME
acetaminophen, dextromethorphan hbr, triprolidine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-963 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 2.5 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color blue Score Shape Size Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-963-45 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/23/2023 Labeler - CVS WOONSOCKET PRESCRIPTION CENTER, INCORPORATED (062312574) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(51316-963) , pack(51316-963)