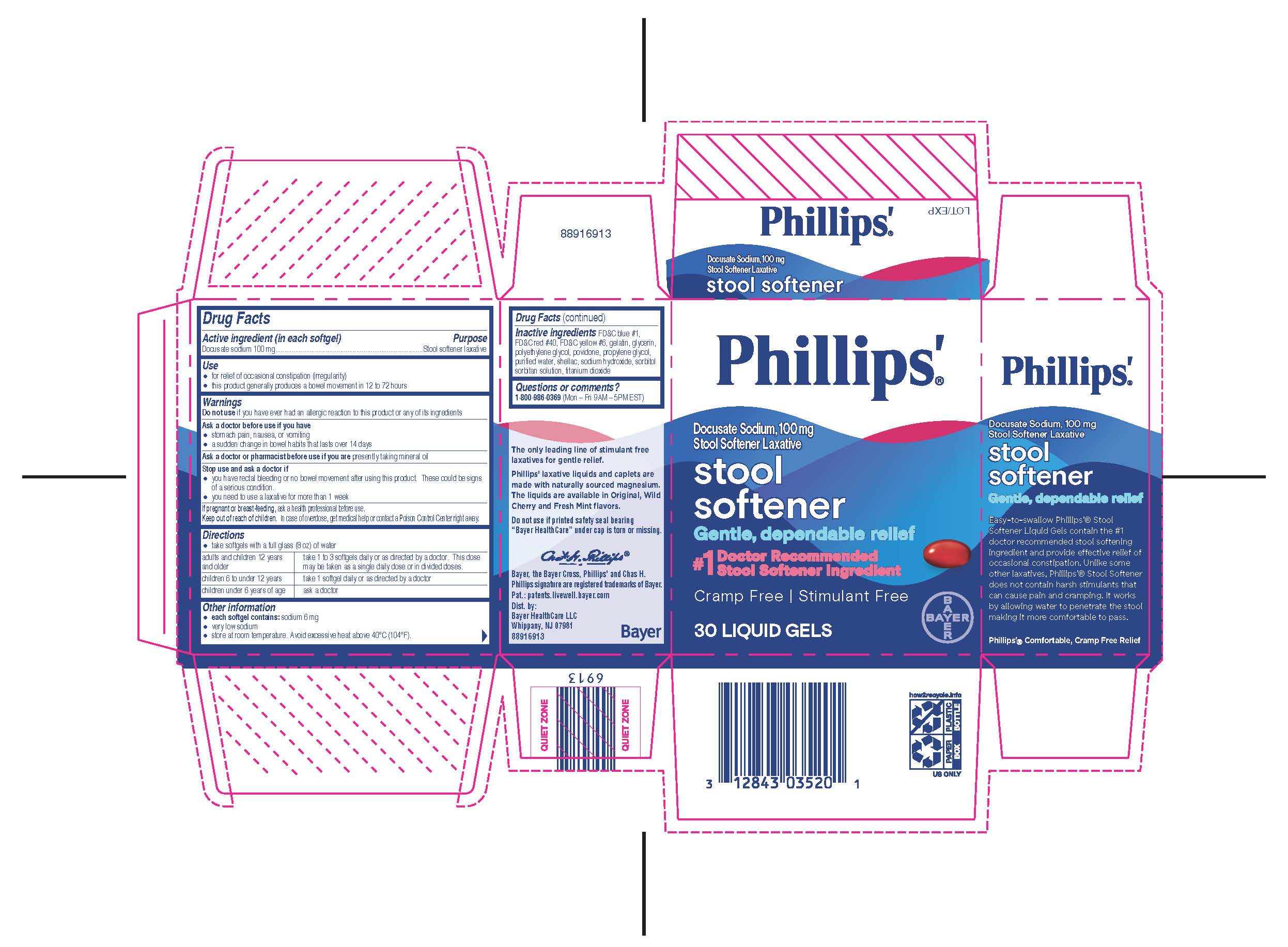
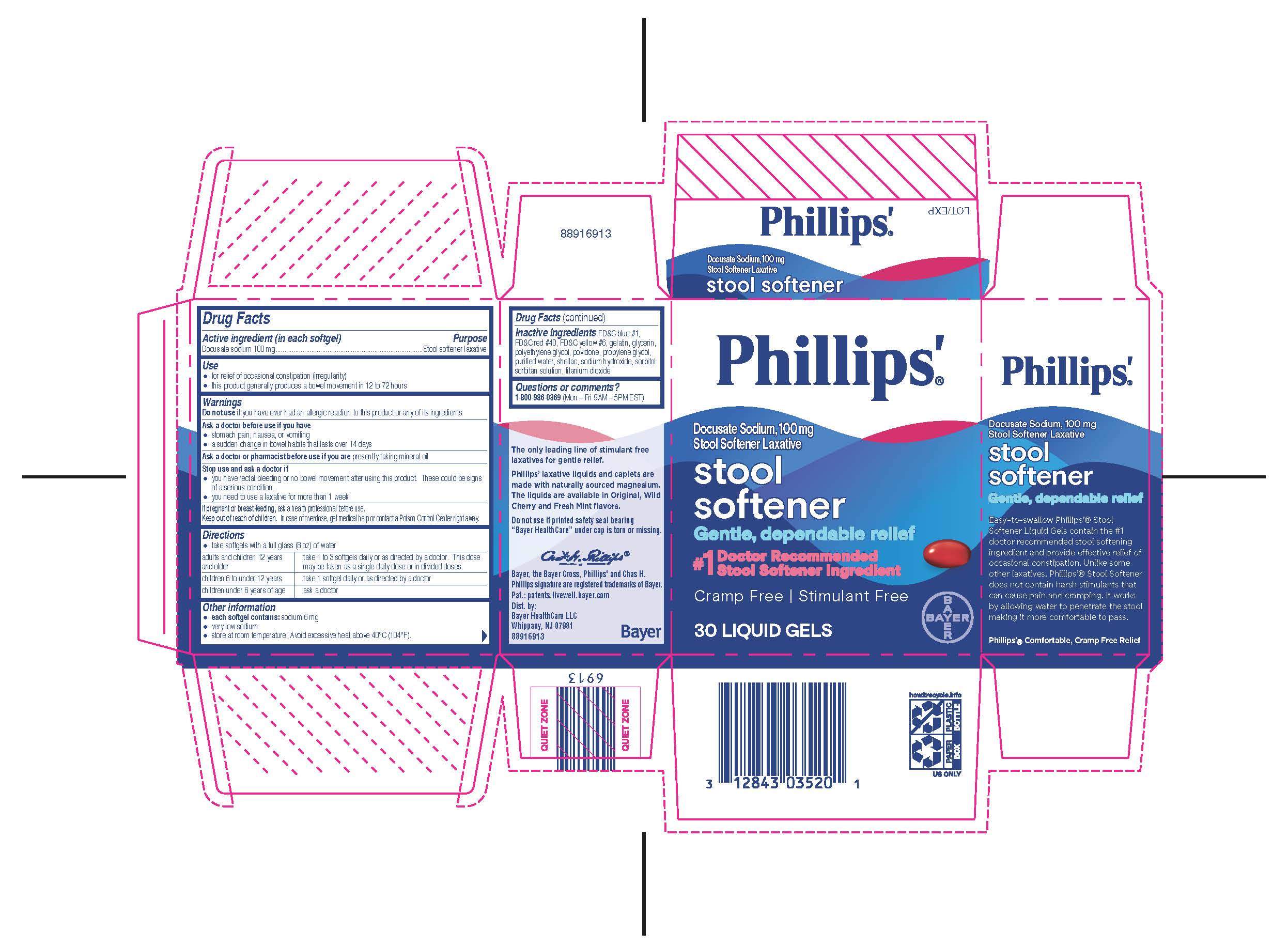
Label: PHILLIPS ORIGINAL MILK OF MAGNESIA liquid
- NDC Code(s): 0280-0030-04, 0280-0030-12, 0280-0030-26
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- Use
- WARNINGS
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
● do not exceed the maximum recommended daily dose in a 24 hour period
● shake well before use
● dose may be taken once a day preferably at bedtime, in divided doses, or as directed by a doctor. Drink a full glass (8 oz) of liquid with each dose.
● for accurate dosing, only use the dosing cup provided
● mL = milliliter
adults and children 12 years and older 30 mL to 60 mL children 6 to 11 years 15 mL to 30 mL children under 6 years ask a doctor - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHILLIPS ORIGINAL MILK OF MAGNESIA
phillips original milk of magnesia liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-0030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 1200 mg in 15 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYPOCHLORITE (UNII: DY38VHM5OD) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-0030-12 355 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/16/2014 2 NDC:0280-0030-26 769 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/16/2014 3 NDC:0280-0030-04 1 in 1 CARTON 12/16/2014 3 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 12/16/2014 Labeler - Bayer HealthCare LLC. (112117283)