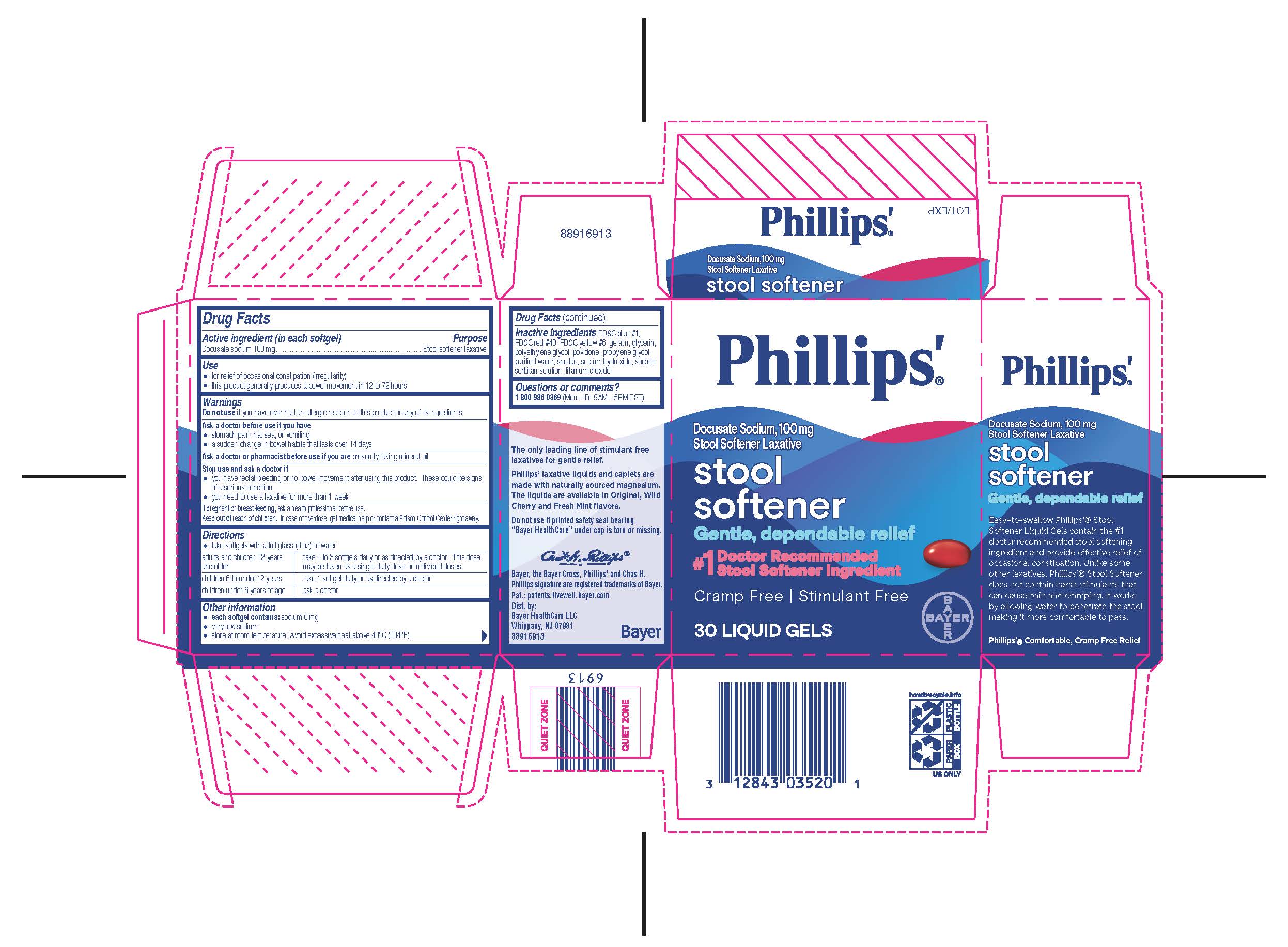
Use
Use relieves occasional constipation (irregularity). This product usually produces bowel movement in ½ to 6 hours.
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
● kidney disease
● a magnesium-restricted diet
● stomach pain, nausea, or vomiting
● a sudden change in bowel habits that lasts over 14 days
Ask a doctor or pharmacist before use if you are taking a prescription drug. This product may interact with certain prescription drugs.
Stop use and ask a doctor if
● you have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
● you need to use a laxative for more than 1 week
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
● do not exceed the maximum recommended daily dose in a 24 hour period
● shake well before use
● dose may be taken once a day preferably at bedtime, in divided doses, or as directed by a doctor. Drink a full glass (8 oz) of liquid with each dose.
● for accurate dosing, only use the dosing cup provided
● mL = milliliter
| adults and children 12 years and older | 30 mL to 60 mL |
| children 6 to 11 years | 15 mL to 30 mL |
| children under 6 years | ask a doctor |