Label: AQUAPHOR HEALING- petrolatum ointment
-
NDC Code(s):
10356-101-02,
10356-101-06,
10356-101-08,
10356-101-10, view more10356-101-23, 10356-101-29, 10356-101-30, 10356-101-35, 10356-101-37, 10356-101-38, 10356-101-39, 10356-101-40, 10356-101-42, 10356-101-43, 10356-101-44, 10356-101-49, 10356-101-50, 10356-101-57
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
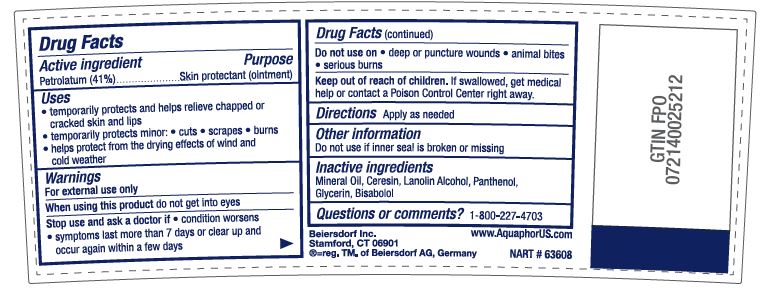
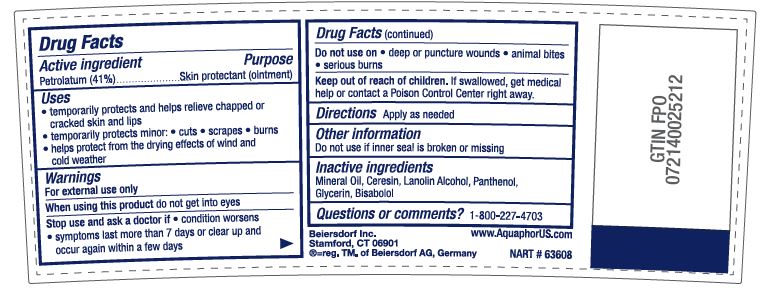
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
• temporarily protects minor: • cuts • scrapes • burns
• temporarily protects and helps relieve chapped or cracked skin and lips
• helps protect from the drying effects of wind and cold weather• helps treat and prevent diaper rash
• protects chafed skin associated with diaper rash and helps protect from wetness - WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- QUESTIONS
- DOSAGE & ADMINISTRATION
-
PRINCIPAL DISPLAY PANEL
Aquaphor Healing Ointment
Number 1 Dermatologist Recommended brand for dry feet and heels
Advanced Therapy
For dry, cracked heels and feet
Clinically proven to restore smooth, healthy looking skin
Skin Protectant
Preservative & Fragrance FreeDermatolgist recommended
Sponge-tip applicator for touch-free application
Recommended by Dermatolgists, Aquaphor Healing Ointment protects to help heal dry, cracked skin on heels ad feet

Aquaphor Healing Ointment
Advanced Therapy
For dry, cracked or irritated skin
Clinically proven to restore smooth, healthy skin
Preservative and Fragrance Free
Dermatologist recommended
Skin Protectant
Number 1 Dermatologist Recommended brand for dry, cracked skin

Aquaphor Healing OintmentBaby
Advanced Therapy
For dry, chapped or irritated skin
Hypoallergenic
Skin Protectant
Fragrance Free
Pediatrician recommended brand
Aquaphor Healing Ointment
Number 1 Dermatologist Recommended brand for minor wound care
Advanced Protection
Soothes minor wouds, cuts, scrapes and burns
Protects skin to enhance healing
Skin Protectant
Preservative and Fragrance Free
Aquaphor Healing Ointment protects the skin to enhance the natural healing process
and help prevent external irritants from reaching the wound

Children's
Aquaphor Healing Ointment
Advanced Therapy
For dry, chapped or irritated skin
Hypoallergenic
Skin Protectant
Fragrance and Preservative Free
Pediatrician recommended bran

-
INGREDIENTS AND APPEARANCE
AQUAPHOR HEALING
petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 41 g in 100 g Inactive Ingredients Ingredient Name Strength CERESIN (UNII: Q1LS2UJO3A) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) GLYCERIN (UNII: PDC6A3C0OX) PANTHENOL (UNII: WV9CM0O67Z) LEVOMENOL (UNII: 24WE03BX2T) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-101-37 396 g in 1 JAR; Type 0: Not a Combination Product 01/01/1991 2 NDC:10356-101-10 99 g in 1 JAR; Type 0: Not a Combination Product 01/01/1991 3 NDC:10356-101-08 85 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1991 4 NDC:10356-101-38 198 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1991 5 NDC:10356-101-06 50 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1991 6 NDC:10356-101-23 10 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1991 7 NDC:10356-101-02 7 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1991 8 NDC:10356-101-29 4 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1991 9 NDC:10356-101-30 0.9 g in 1 PACKET; Type 0: Not a Combination Product 01/01/1991 10 NDC:10356-101-39 7 g in 1 JAR; Type 0: Not a Combination Product 01/01/1991 11 NDC:10356-101-40 2 in 1 CARTON 01/01/1991 11 NDC:10356-101-23 10 g in 1 TUBE; Type 0: Not a Combination Product 12 NDC:10356-101-42 1.7 g in 1 PACKET; Type 0: Not a Combination Product 09/01/2018 13 NDC:10356-101-43 250 in 1 CARTON 09/01/2018 13 NDC:10356-101-42 1.7 g in 1 PACKET; Type 0: Not a Combination Product 14 NDC:10356-101-44 144 in 1 CARTON 01/01/1991 14 NDC:10356-101-30 0.9 g in 1 PACKET; Type 0: Not a Combination Product 15 NDC:10356-101-35 141 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2020 16 NDC:10356-101-49 297 g in 1 JAR; Type 0: Not a Combination Product 09/01/1991 17 NDC:10356-101-50 43 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2020 18 NDC:10356-101-57 80 g in 1 JAR; Type 0: Not a Combination Product 01/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 01/01/1991 Labeler - Beiersdorf Inc (001177906)