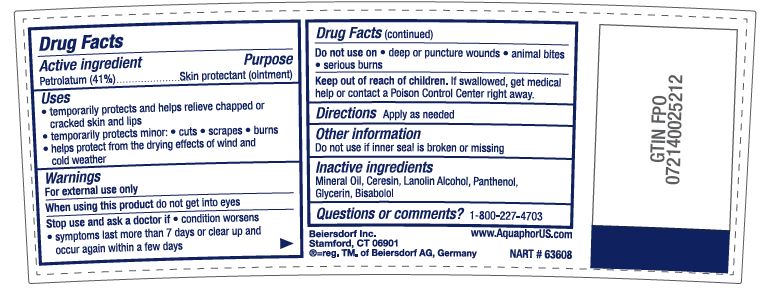
Uses
• temporarily protects minor: • cuts • scrapes • burns
• temporarily protects and helps relieve chapped or cracked skin and lips
• helps protect from the drying effects of wind and cold weather
• helps treat and prevent diaper rash
• protects chafed skin associated with diaper rash and helps protect from wetness
Stop use and ask a doctor if • condition worsens
• symptoms last more than 7 days or clear up and occur
again within a few days
Keep out of reach of children. If swallowed, get medical help
or contact a Poison Control Center right away.
Directions
• apply as needed
• for diaper rash, change wet and soiled diapers promptly, cleanse the diaper area, and allow to dry. Apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
Aquaphor Healing Ointment
Number 1 Dermatologist Recommended brand for dry feet and heels
Advanced Therapy
For dry, cracked heels and feet
Clinically proven to restore smooth, healthy looking skin
Skin Protectant
Preservative & Fragrance Free
Dermatolgist recommended
Sponge-tip applicator for touch-free application
Recommended by Dermatolgists, Aquaphor Healing Ointment protects to help heal dry, cracked skin on heels ad feet

Aquaphor Healing Ointment
Advanced Therapy
For dry, cracked or irritated skin
Clinically proven to restore smooth, healthy skin
Preservative and Fragrance Free
Dermatologist recommended
Skin Protectant
Number 1 Dermatologist Recommended brand for dry, cracked skin

Aquaphor Healing Ointment
Baby
Advanced Therapy
For dry, chapped or irritated skin
Hypoallergenic
Skin Protectant
Fragrance Free
Pediatrician recommended brand

Aquaphor Healing Ointment
Number 1 Dermatologist Recommended brand for minor wound care
Advanced Protection
Soothes minor wouds, cuts, scrapes and burns
Protects skin to enhance healing
Skin Protectant
Preservative and Fragrance Free
Aquaphor Healing Ointment protects the skin to enhance the natural healing process
and help prevent external irritants from reaching the wound

Children's
Aquaphor Healing Ointment
Advanced Therapy
For dry, chapped or irritated skin
Hypoallergenic
Skin Protectant
Fragrance and Preservative Free
Pediatrician recommended bran
