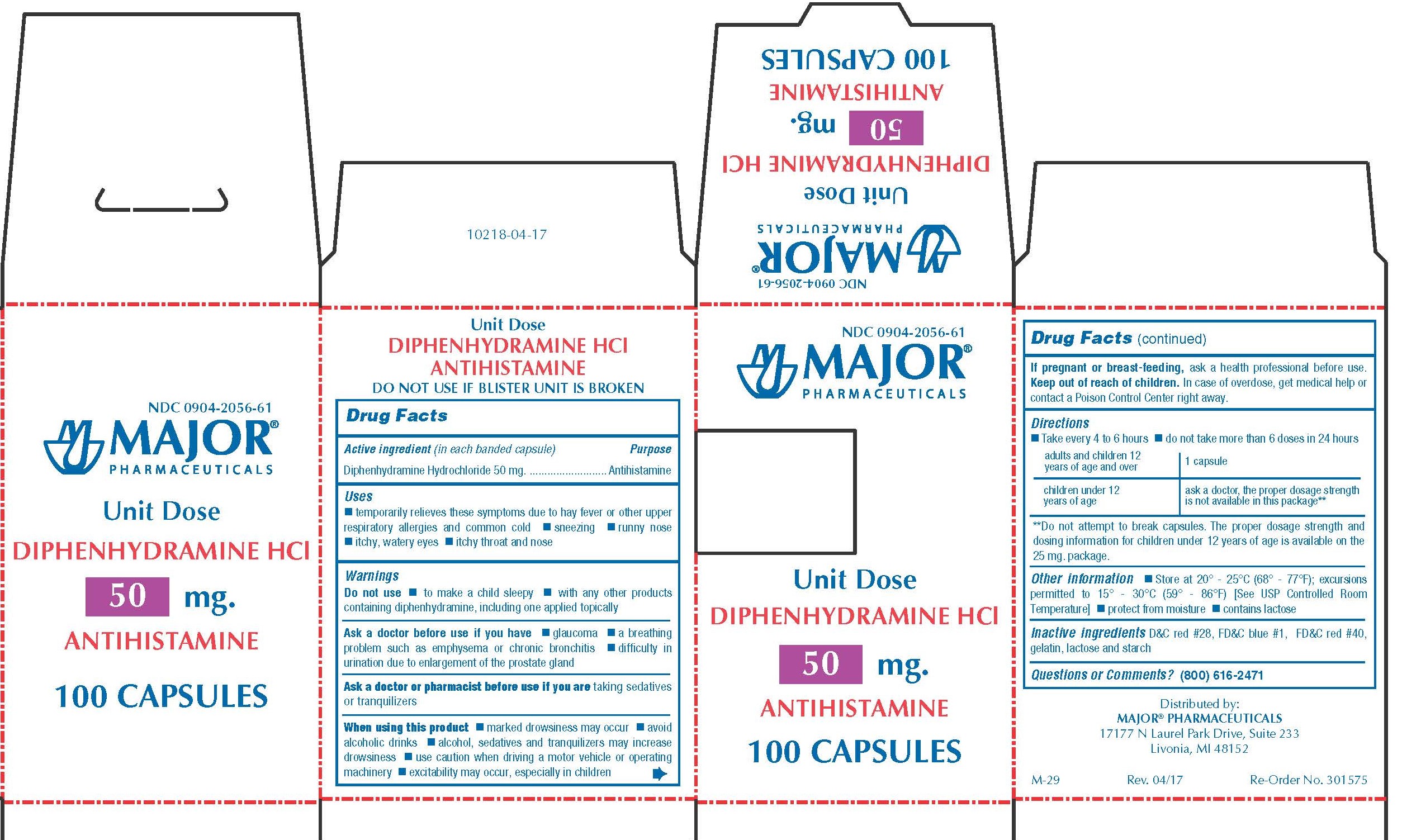
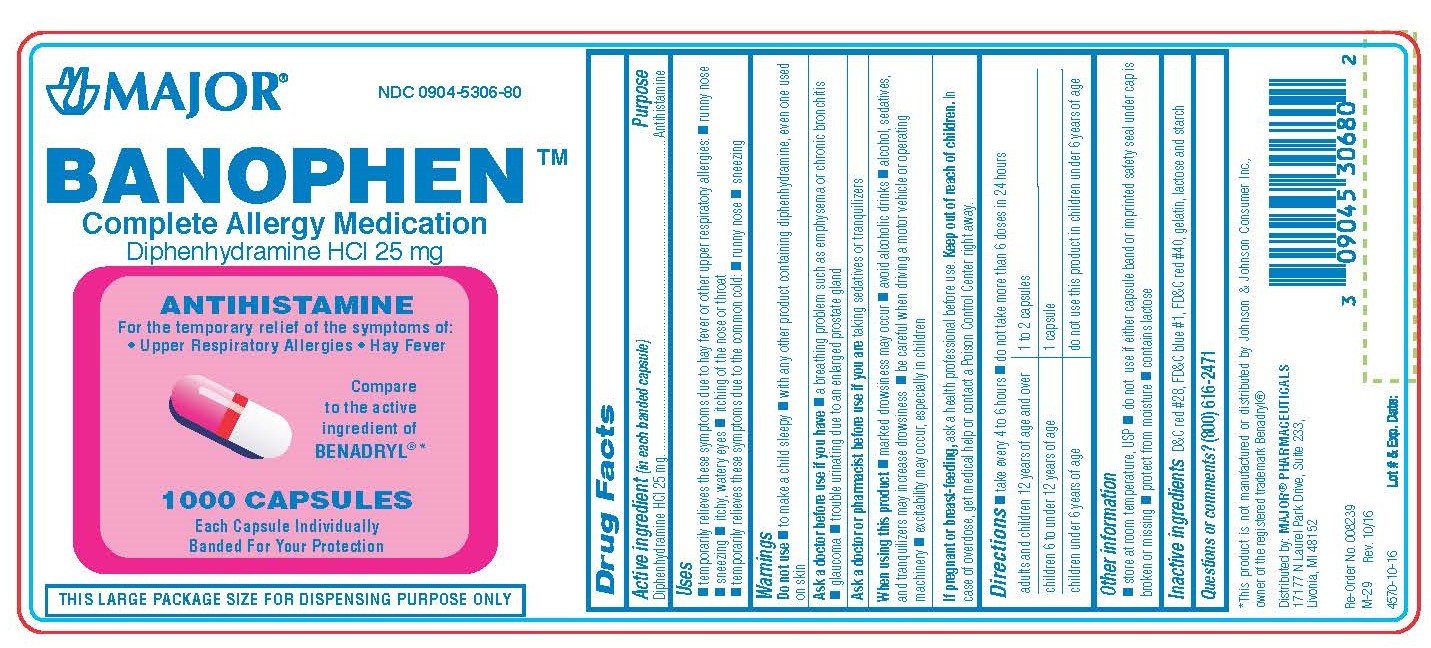
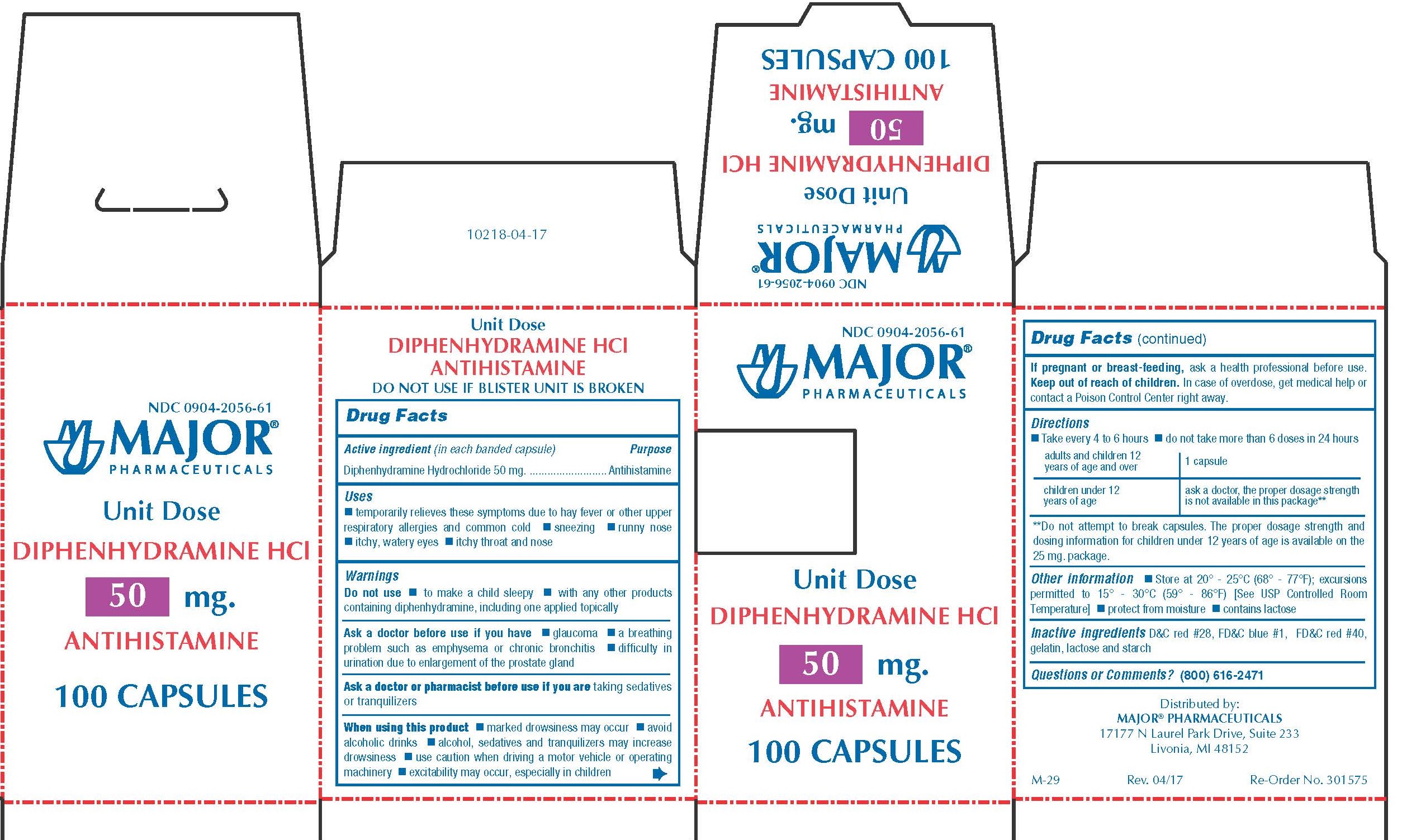
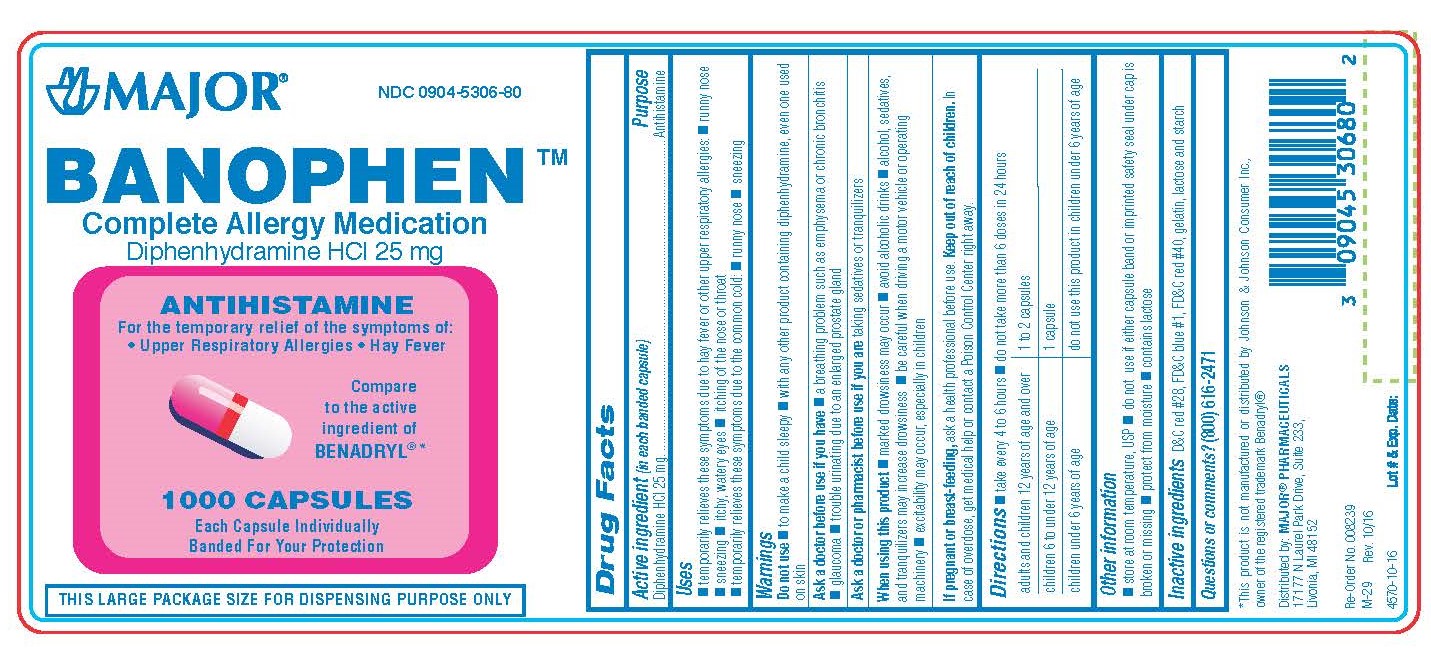
Label: DIPHENHYDRAMINE HYDROCHLORIDE capsule
BANOPHEN- diphenhydramine hcl capsule
-
NDC Code(s):
0904-2035-24,
0904-2056-61,
0904-5306-24,
0904-5306-60, view more0904-5306-61, 0904-5306-80
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 6, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each banded capsule)
- Purpose
-
Use
25 MG
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat and nose
- Temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
50 MG
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies and common cold
- sneezing
- runny nose
- itchy, watery eyes
- itchy throat and nose
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- WARNINGS
- Ask a doctor before use if you have
- Ask a doctor or pharmacist
- When using this product
- If pregnant or breast-feeding
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Take every 4-6 hours
- Do not take more than 6 doses in 24 hours
25 MG
adults and children 12 years of age and over 1 to 2 capsules children 6 years to under 12 years of age 1 capsule children under 6 years of age do not use this product in children under 6 years of age 50 MG
adults and children 12 years of age and over 1 capsule children 6 years to under 12 years of age Ask a doctor, the proper dosage strength is not available in this package** **Do not attempt to break capsules. The proper dosage strength and dosing information for children under 12 years of age is available on the 25 mg package.
- Other Information
- Inactive Ingredients
- Questions?
- Distributed by
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- Major-24BB
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-5306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink (half pink and half clear with white powder inside) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CPC;835 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-5306-60 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/02/2009 2 NDC:0904-5306-80 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/02/2009 3 NDC:0904-5306-61 10 in 1 BOX 01/02/2009 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:0904-5306-24 2 in 1 CARTON 03/15/2019 4 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/02/2009 DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-2056 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CPC;836 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-2056-61 10 in 1 BOX 01/02/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/02/2009 BANOPHEN
diphenhydramine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-2035 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink (half pink and half clear with white powder inside) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CPC;835 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-2035-24 2 in 1 CARTON 01/02/2009 08/31/2021 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/02/2009 Labeler - Major Pharmaceuticals (191427277)