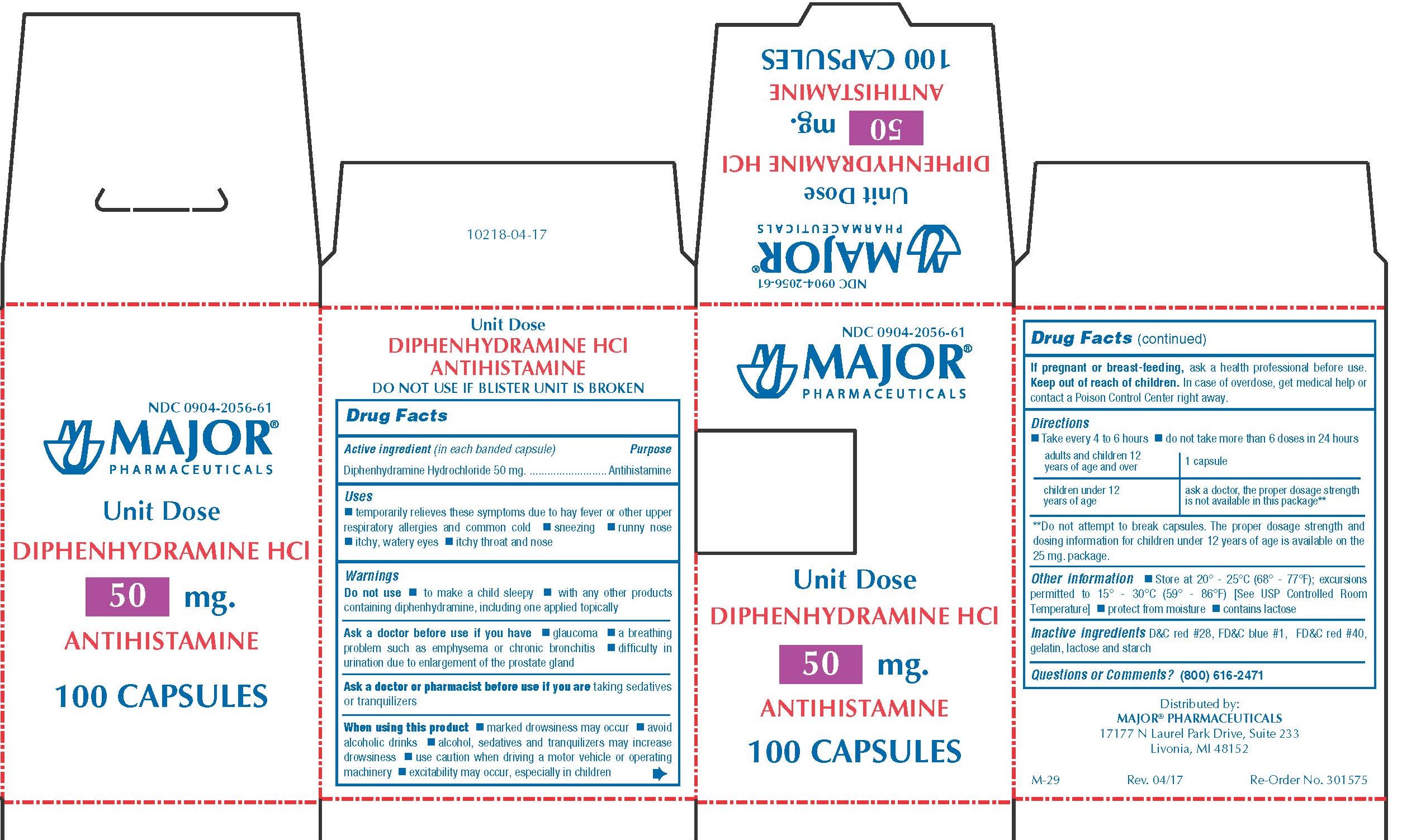
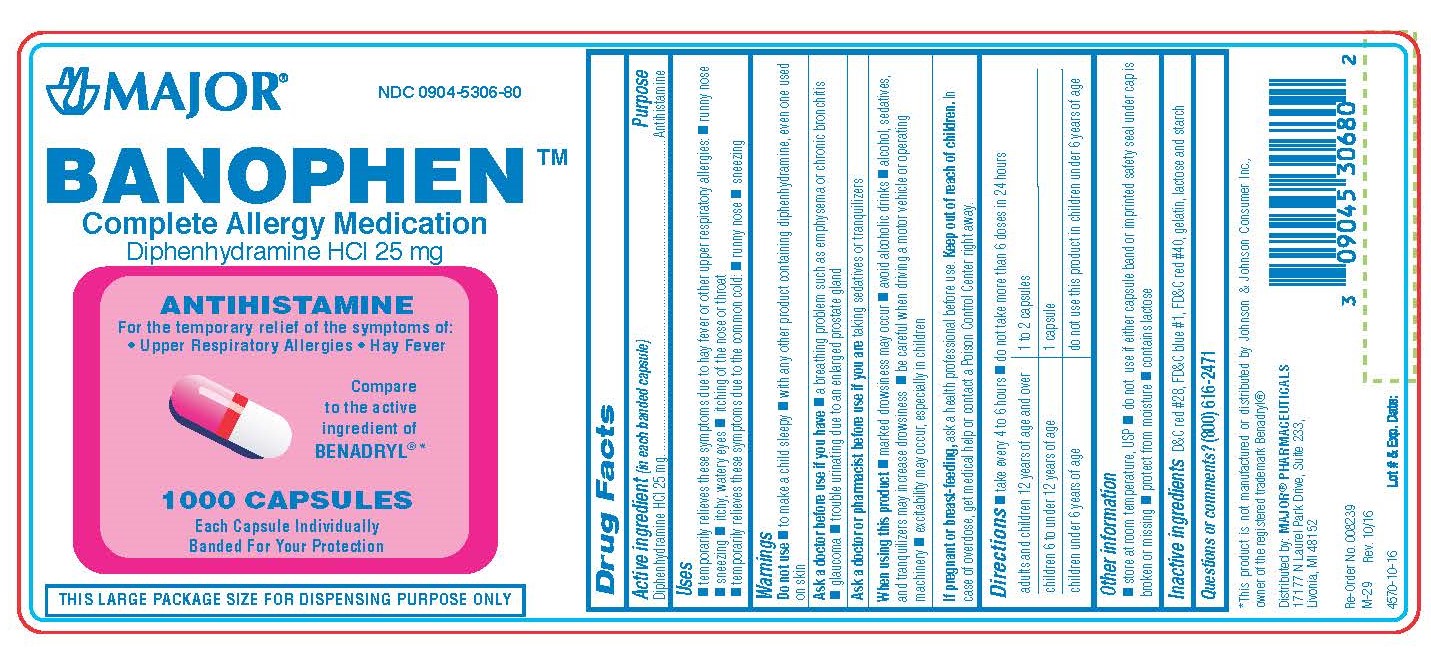
Use
25 MG
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat and nose
- Temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
50 MG
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies and common cold
- sneezing
- runny nose
- itchy, watery eyes
- itchy throat and nose
WARNINGS
Do not use
25 MG
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
50 MG
- to make a child sleepy
- with any other product containing diphenhydramine, including one applied topically
Ask a doctor before use if you have
25 MG
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
50 MG
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Take every 4-6 hours
- Do not take more than 6 doses in 24 hours
25 MG
| adults and children 12 years of age and over | 1 to 2 capsules |
| children 6 years to under 12 years of age | 1 capsule |
| children under 6 years of age | do not use this product in children under 6 years of age |
50 MG
| adults and children 12 years of age and over | 1 capsule |
| children 6 years to under 12 years of age | Ask a doctor, the proper dosage strength is not available in this package** |
**Do not attempt to break capsules. The proper dosage strength and dosing information for children under 12 years of age is available on the 25 mg package.