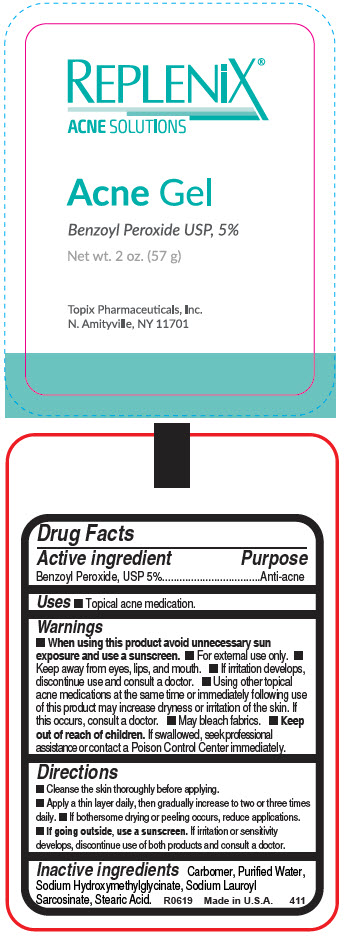
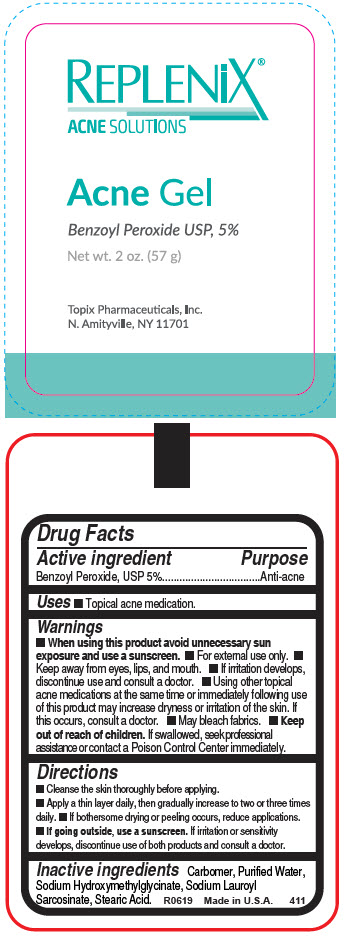
Label: REPLENIX ACNE- benzoyl peroxide gel
- NDC Code(s): 51326-411-57
- Packager: Topiderm, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Cleanse the skin thoroughly before applying.
- Apply a thin layer daily, then gradually increase to two or three times daily.
- If bothersome drying or peeling occurs, reduce applications.
- If going outside, use a sunscreen. If irritation or sensitivity develops, discontinue use of both products and consult a doctor.
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 57 g Tube Label
-
INGREDIENTS AND APPEARANCE
REPLENIX ACNE
benzoyl peroxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51326-411 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) SODIUM HYDROXYMETHYLGLYCINATE (UNII: DIG6BWZ9XT) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51326-411-57 57 g in 1 TUBE; Type 0: Not a Combination Product 01/22/1993 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M006 01/22/1993 Labeler - Topiderm, Inc (049121643) Registrant - Topiderm, Inc. (049121643) Establishment Name Address ID/FEI Business Operations Topiderm, Inc 049121643 MANUFACTURE(51326-411) Establishment Name Address ID/FEI Business Operations Topix Pharmaceuticals, Inc. 117745066 PACK(51326-411)