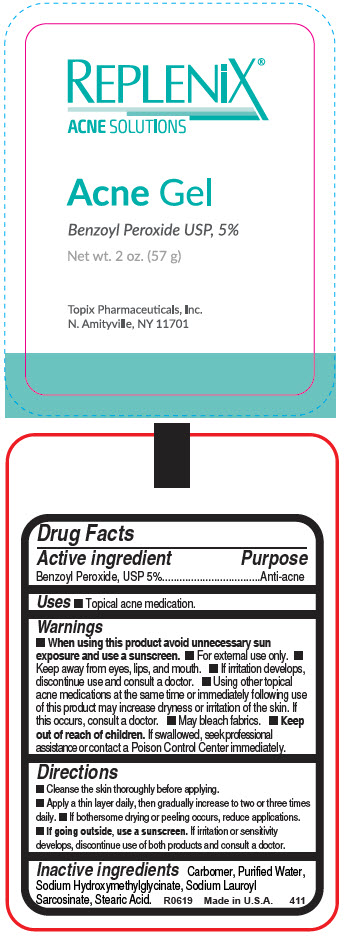
Active ingredient
Benzoyl Peroxide, USP 5%
Warnings
-
When using this product avoid unnecessary sun exposure and use a sunscreen.
- For external use only.
- Keep away from eyes, lips, and mouth.
- If irritation develops, discontinue use and consult a doctor.
- Using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, consult a doctor.
- May bleach fabrics.
-
Keep out of reach of children. If swallowed, seek professional assistance or contact a Poison Control Center immediately.
Directions
- Cleanse the skin thoroughly before applying.
- Apply a thin layer daily, then gradually increase to two or three times daily.
- If bothersome drying or peeling occurs, reduce applications.
-
If going outside, use a sunscreen. If irritation or sensitivity develops, discontinue use of both products and consult a doctor.
Inactive ingredients
Carbomer, Purified Water, Sodium Hydroxymethylglycinate, Sodium Lauroyl Sarcosinate, Stearic Acid.
PRINCIPAL DISPLAY PANEL - 57 g Tube Label
REPLENiX®
ACNE SOLUTIONS
Acne Gel
Benzoyl Peroxide USP, 5%
Net wt. 2 oz. (57 g)
Topix Pharmaceuticals, Inc.
N. Amityville, NY 11701