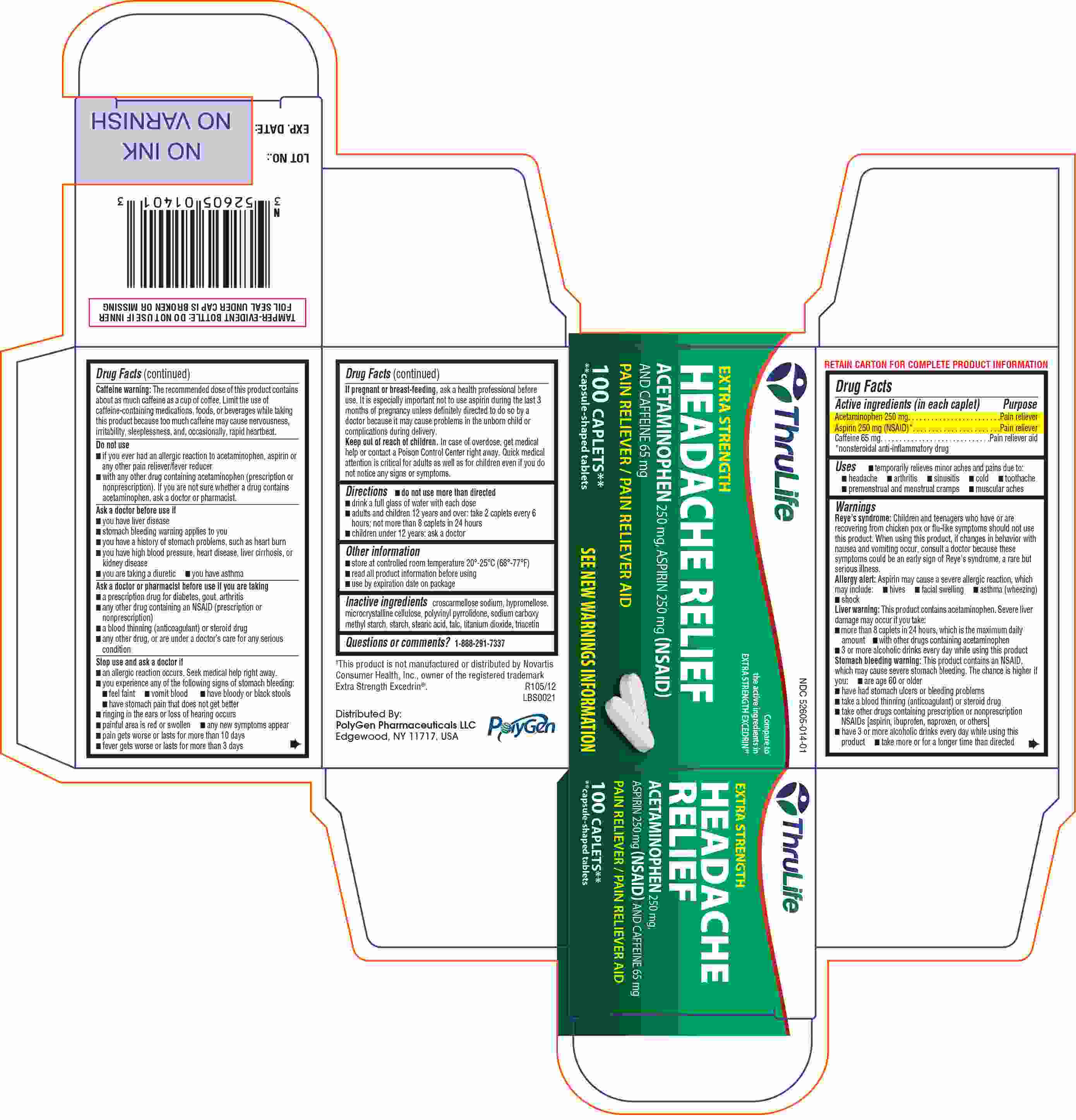
Label: THRULIFE EXTRA STRENGTH HEADACHE RELIEF- acetaminophen, aspirin, caffeine tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 52605-014-01 - Packager: Polygen Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 12, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
- Purpose
- Use(s)
-
Warnings
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or flu like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives · facial swelling ·asthma (wheezing) · shock
Liver Warning: This product contains acetaminophen.
Severe liver damage may occur if you take · more than 8 tablets in 24 hours, which is the maximum daily amount · with other drugs containing acetaminophen · 3 or more alcoholic drinks every day while using this product.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you ·are age 60 or older ·have had stomach ulcers or bleeding problems · take a blood thinning (anticoagulant) or steroid drug ·take other drugs containing prescription or nonprescription NSAIDs ( aspirin, ibuprofen, naproxen, or others) ·have 3 or more alcoholic drinks every day while using this product ·take more or for a longer time than directed.
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and occasionally, rapid heartbeat.
- Do not use
- Ask a doctor before use if
- Ask a doctor or pharmacist before use if
-
Stop use and ask doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
· feel faint · vomit blood · have bloody or black stools ·have stomach pain that does not get better.
- painful area is red or swollen
- any few symptoms appear ·ringing in the ears or loss of hearing occurs.
●pain gets worse or lasts for more than 10 days ● fever gets worse or lasts for more than 3 days
- Pregnancy/Breastfeeding
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Dosage & Administration section
- OTC - Questions section
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
THRULIFE EXTRA STRENGTH HEADACHE RELIEF
acetaminophen, aspirin, caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52605-014 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 18mm Flavor Imprint Code P14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52605-014-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part343 01/31/2013 Labeler - Polygen Pharmaceuticals LLC (962415720) Establishment Name Address ID/FEI Business Operations Polygen Pharmaceuticals LLC 962415720 RELABEL(52605-014) , REPACK(52605-014) , MANUFACTURE(52605-014) , PACK(52605-014)