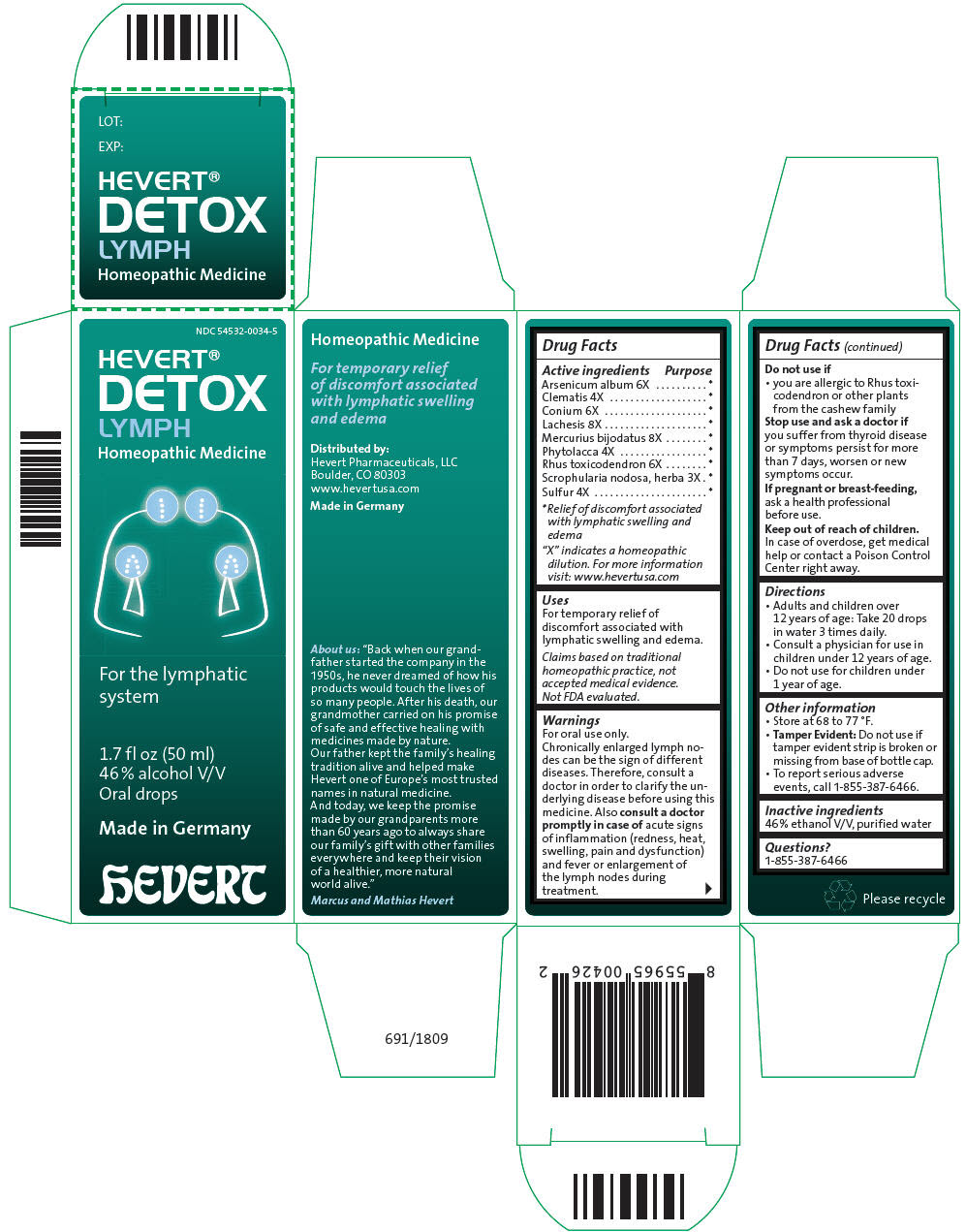
Label: HEVERT DETOX LYMPH- arsenic trioxide, clematis recta flowering top, conium maculatum flowering top, lachesis muta venom, mercuric iodide, phytolacca americana root, toxicodendron pubescens shoot, scrophularia nodosa leaf with stem, and sulfur liquid
- NDC Code(s): 54532-0034-5
- Packager: Hevert Arzneimittel GmbH & Co KG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients Purpose "X" indicates a homeopathic dilution. For more information visit: www.hevertusa.com - *
- Relief of discomfort associated with lymphatic swelling and edema
Arsenicum album 6X * Clematis 4X * Conium 6X * Lachesis 8X * Mercurius bijodatus 8X * Phytolacca 4X * Rhus toxicodendron 6X * Scrophularia nodosa, herba 3X * Sulfur 4X * - Uses
-
Warnings
For oral use only.
Chronically enlarged lymph nodes can be the sign of different diseases. Therefore, consult a doctor in order to clarify the underlying disease before using this medicine. Also consult a doctor promptly in case of acute signs of inflammation (redness, heat, swelling, pain and dysfunction) and fever or enlargement of the lymph nodes during treatment.
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
HEVERT DETOX LYMPH
arsenic trioxide, clematis recta flowering top, conium maculatum flowering top, lachesis muta venom, mercuric iodide, phytolacca americana root, toxicodendron pubescens shoot, scrophularia nodosa leaf with stem, and sulfur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54532-0034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Arsenic Trioxide (UNII: S7V92P67HO) (Arsenic Cation (3+) - UNII:C96613F5AV) Arsenic Trioxide 6 [hp_X] in 50 mL Clematis Recta Flowering top (UNII: 396421SP9F) (Clematis Recta Flowering top - UNII:396421SP9F) Clematis Recta Flowering top 4 [hp_X] in 50 mL Conium Maculatum Flowering Top (UNII: Q28R5GF371) (Conium Maculatum Flowering Top - UNII:Q28R5GF371) Conium Maculatum Flowering Top 6 [hp_X] in 50 mL Lachesis Muta Venom (UNII: VSW71SS07I) (Lachesis Muta Venom - UNII:VSW71SS07I) Lachesis Muta Venom 8 [hp_X] in 50 mL Mercuric Iodide (UNII: R03O05RB0P) (Mercuric Iodide - UNII:R03O05RB0P) Mercuric Iodide 8 [hp_X] in 50 mL Phytolacca Americana Root (UNII: 11E6VI8VEG) (Phytolacca Americana Root - UNII:11E6VI8VEG) Phytolacca Americana Root 4 [hp_X] in 50 mL Toxicodendron Pubescens shoot (UNII: 46PYZ1F82M) (Toxicodendron Pubescens shoot - UNII:46PYZ1F82M) Toxicodendron Pubescens shoot 6 [hp_X] in 50 mL Scrophularia Nodosa leaf with stem (UNII: K93UPA2CNQ) (Scrophularia Nodosa leaf with stem - UNII:K93UPA2CNQ) Scrophularia Nodosa leaf with stem 3 [hp_X] in 50 mL Sulfur (UNII: 70FD1KFU70) (Sulfur - UNII:70FD1KFU70) Sulfur 4 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54532-0034-5 1 in 1 CARTON 06/15/2016 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/15/2016 Labeler - Hevert Arzneimittel GmbH & Co KG (318100617) Establishment Name Address ID/FEI Business Operations Hevert Arzneimittel GmbH & Co. KG 318100617 MANUFACTURE(54532-0034)