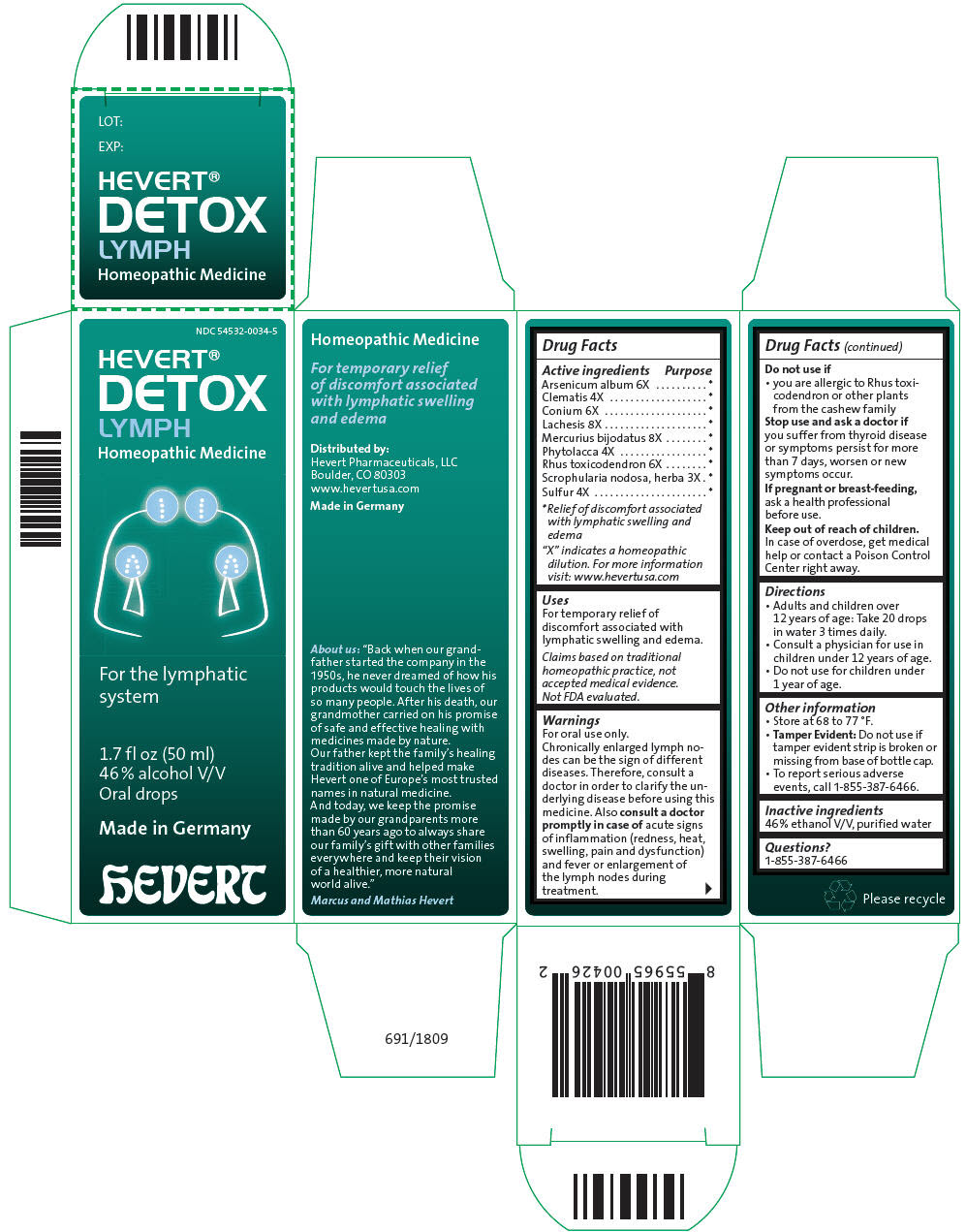
HEVERT DETOX LYMPH- arsenic trioxide, clematis recta flowering top, conium maculatum flowering top, lachesis muta venom, mercuric iodide, phytolacca americana root, toxicodendron pubescens shoot, scrophularia nodosa leaf with stem, and sulfur liquid
Hevert Arzneimittel GmbH & Co KG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Active ingredients | Purpose |
| "X" indicates a homeopathic dilution. For more information visit: www.hevertusa.com |
|
|
| Arsenicum album 6X | * |
| Clematis 4X | * |
| Conium 6X | * |
| Lachesis 8X | * |
| Mercurius bijodatus 8X | * |
| Phytolacca 4X | * |
| Rhus toxicodendron 6X | * |
| Scrophularia nodosa, herba 3X | * |
| Sulfur 4X | * |
Uses
For temporary relief of discomfort associated with lymphatic swelling and edema.
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings
For oral use only.
Chronically enlarged lymph nodes can be the sign of different diseases. Therefore, consult a doctor in order to clarify the underlying disease before using this medicine. Also consult a doctor promptly in case of acute signs of inflammation (redness, heat, swelling, pain and dysfunction) and fever or enlargement of the lymph nodes during treatment.
Do not use if
- you are allergic to Rhus toxicodendron or other plants from the cashew family
Stop use and ask a doctor if you suffer from thyroid disease or symptoms persist for more than 7 days, worsen or new symptoms occur.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children over 12 years of age: Take 20 drops in water 3 times daily.
- Consult a physician for use in children under 12 years of age.
- Do not use for children under 1 year of age.
Other information
- Store at 68 to 77 °F.
-
Tamper Evident: Do not use if tamper evident strip is broken or missing from base of bottle cap.
- To report serious adverse events, call 1-855-387-6466.
Inactive ingredients
46 % ethanol V/V, purified water
Questions?
1-855-387-6466
Distributed by:
Hevert Pharmaceuticals, LLC
Boulder, CO 80303
PRINCIPAL DISPLAY PANEL - 50 ml Bottle Carton
NDC 54532-0034-5
HEVERT®
DETOX
LYMPH
Homeopathic Medicine
For the lymphatic
system
1.7 fl oz (50 ml)
46 % alcohol V/V
Oral drops
Made in Germany
hEVERT