Label: UVA/UVB SPF 15 RODAN AND FIELDS- octinoxate, zinc oxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 14222-2022-1, 14222-2022-2 - Packager: Rodan & Fields LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
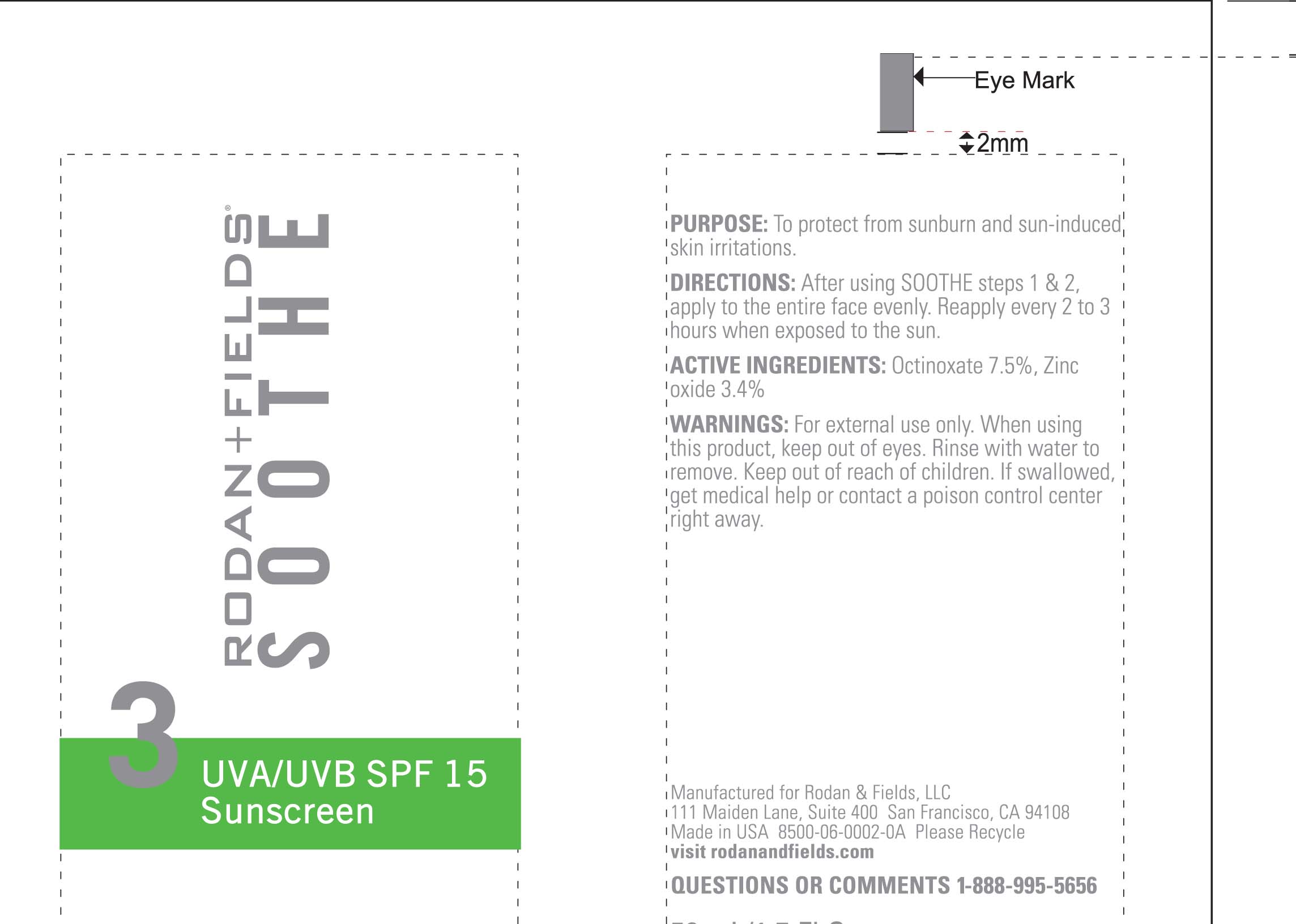
ACTIVE INGREDIENT
Active Ingredients Purpose
Octinoxate 7.5% Sunscreen
Zinc Oxide 3.4% Sunscreen
Uses
Helps prevent sunburn
Higher SPF gives more sun protection
Temporarily protects chapped or cracked skin.
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center immediately.
Directions
Apply a generous dime-sized amount evenly over entire face every morning
Children under 6 months of age: ask a doctor.
Reapply as needed or after towel drying, swimming, or sweating.
Water, Octyldodecanol, Hydrogenated polydecene, butylene glycol, dimethicone, sodium dihydroxycetyl phosphate, glyceryl stearate, PEG-100 stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, astrocaryum murumuru butter, Chamomilla recutita (matricaria) flower oil, Cucumis melo (melon) fruit extract, Persea Gratissima (avacado) oil, Cholesterol/Potassium sulfate, Acetyl glucosamine, squalene, Cholesterol, Linoleic acid, Bisabolol, Tocopheryl Acetate, Oleth-3 phosphate, propylene glycol dicaprylate ethylhexylglycerin, caprylyl glycol, triethoxycaprylylsilane, polysorbate 60, boron nitride, xanthan gum, tetrasodium EDTA, citric acid, potassium sorbate, phenoxyethanol
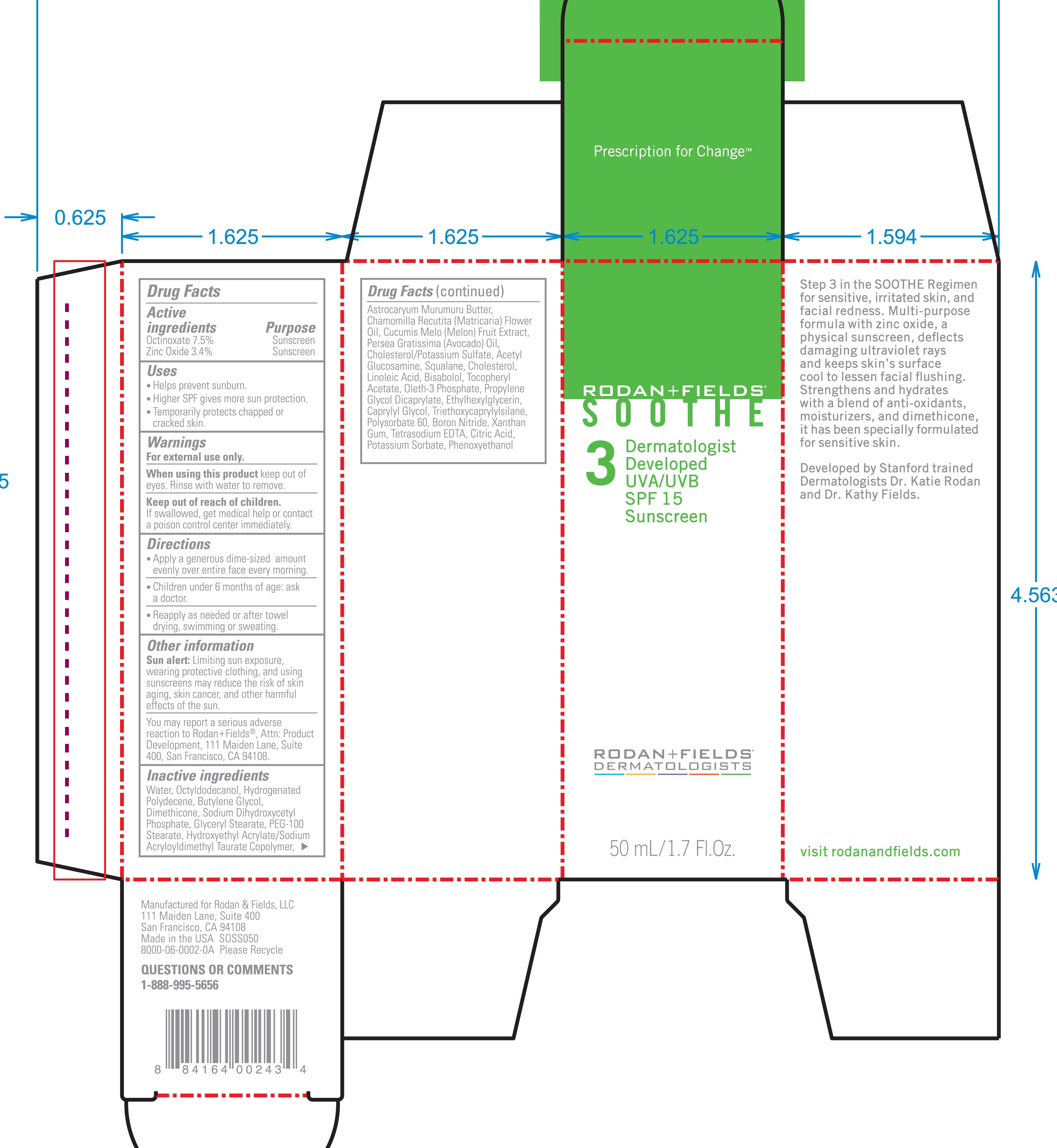
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UVA/UVB SPF 15 RODAN AND FIELDS
octinoxate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mL in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 3.4 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ASTROCARYUM MURUMURU SEED BUTTER (UNII: 12V64UPU6R) CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) CANTALOUPE (UNII: 8QF5D5H6UH) SQUALENE (UNII: 7QWM220FJH) CHOLESTEROL (UNII: 97C5T2UQ7J) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2022-2 1 in 1 BOX 1 NDC:14222-2022-1 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/03/2011 Labeler - Rodan & Fields LLC. (051659584) Registrant - Cosmetic Enterprises Ltd. (017701475) Establishment Name Address ID/FEI Business Operations Cosmetic Enterprises Ltd. 017701475 manufacture