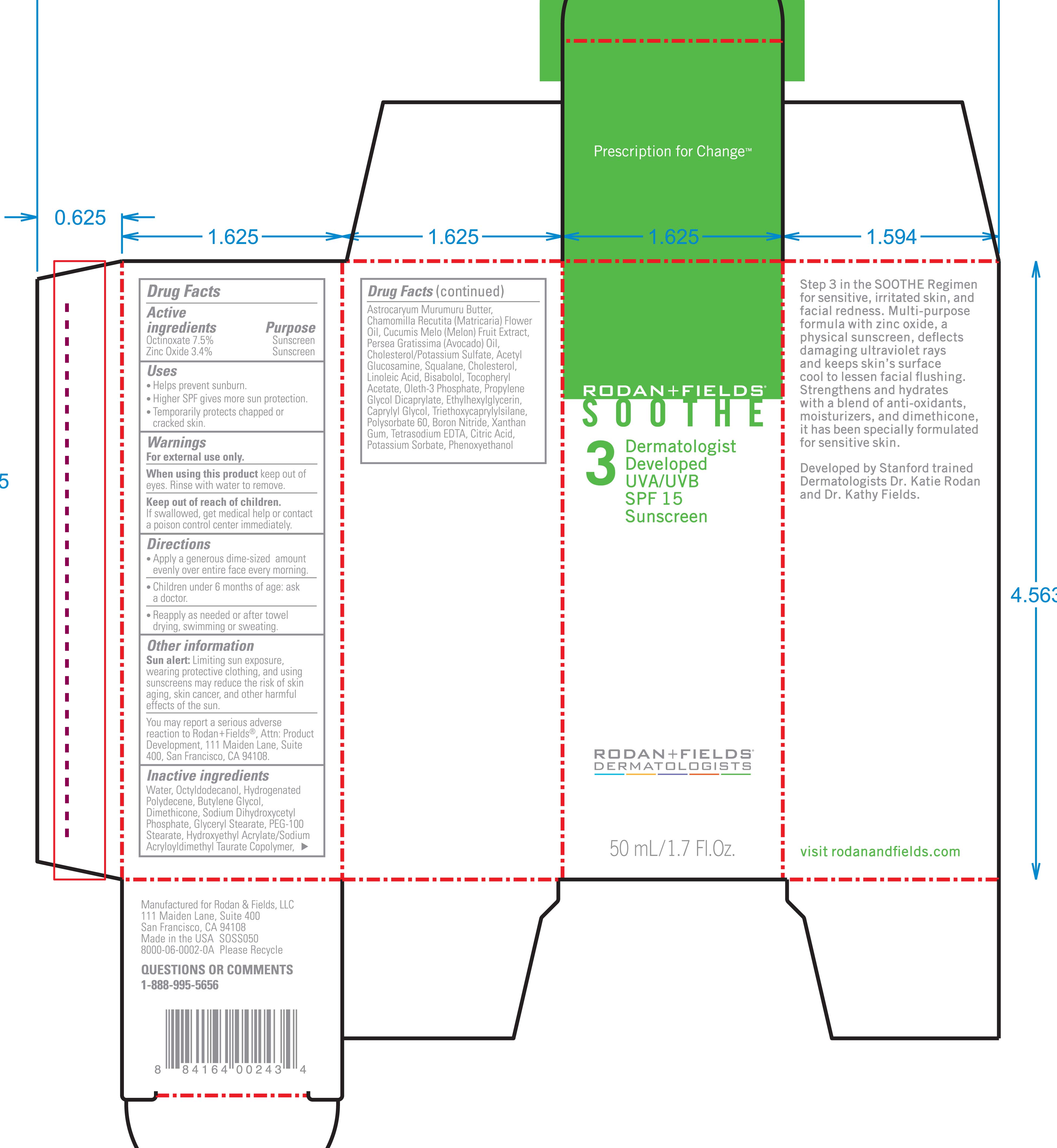
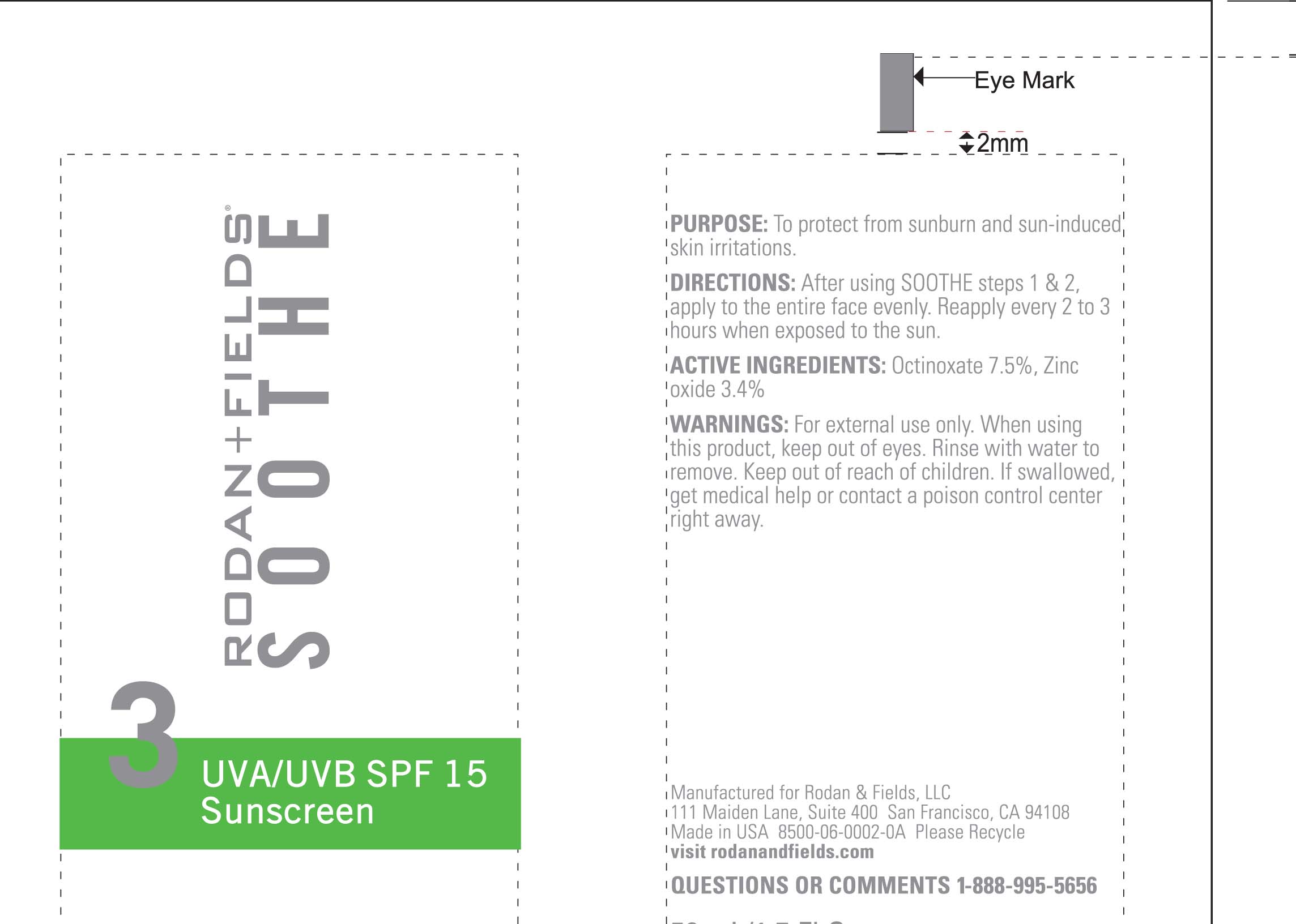
Active Ingredients Purpose
Octinoxate 7.5% Sunscreen
Zinc Oxide 3.4% Sunscreen
Uses
Helps prevent sunburn
Higher SPF gives more sun protection
Temporarily protects chapped or cracked skin.
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center immediately.
Directions
Apply a generous dime-sized amount evenly over entire face every morning
Children under 6 months of age: ask a doctor.
Reapply as needed or after towel drying, swimming, or sweating.
Water, Octyldodecanol, Hydrogenated polydecene, butylene glycol, dimethicone, sodium dihydroxycetyl phosphate, glyceryl stearate, PEG-100 stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, astrocaryum murumuru butter, Chamomilla recutita (matricaria) flower oil, Cucumis melo (melon) fruit extract, Persea Gratissima (avacado) oil, Cholesterol/Potassium sulfate, Acetyl glucosamine, squalene, Cholesterol, Linoleic acid, Bisabolol, Tocopheryl Acetate, Oleth-3 phosphate, propylene glycol dicaprylate ethylhexylglycerin, caprylyl glycol, triethoxycaprylylsilane, polysorbate 60, boron nitride, xanthan gum, tetrasodium EDTA, citric acid, potassium sorbate, phenoxyethanol