Label: CHLORHEXIDINE GLUCONATE SOLUTION 0.75% ANTISEPTIC solution
-
NDC Code(s):
61037-414-01,
61037-414-02,
61037-414-03,
61037-414-04, view more61037-414-05, 61037-414-06, 61037-414-07, 61037-414-08
- Packager: Bajaj Medical, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 26, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
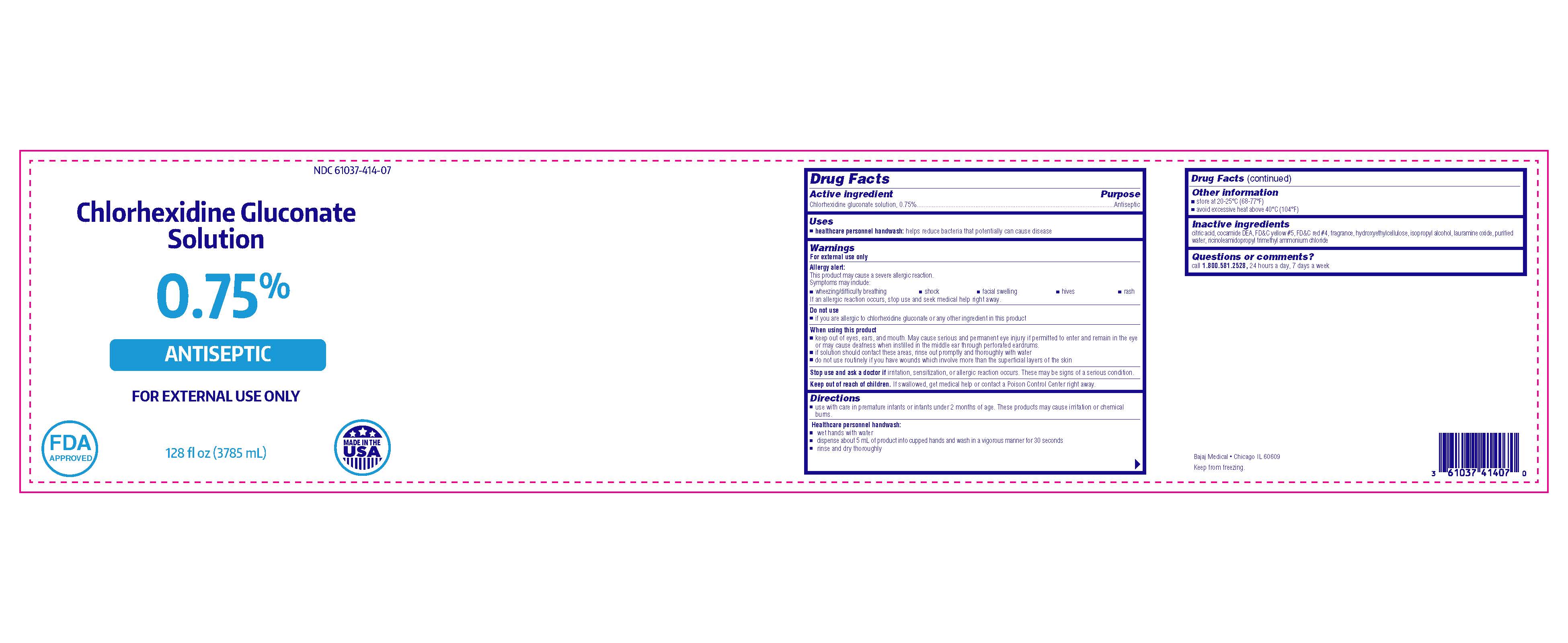
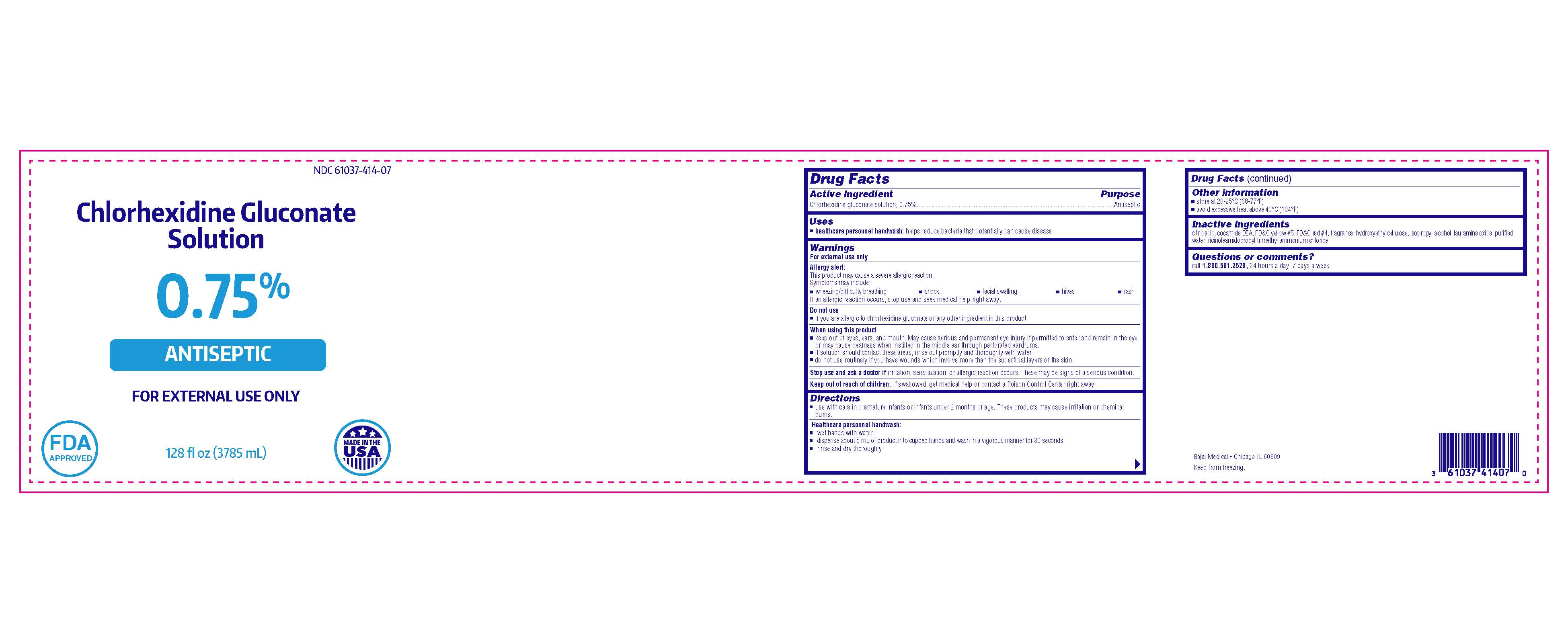
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Allergy alert:
This product may cause a severe allergic reaction.
Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
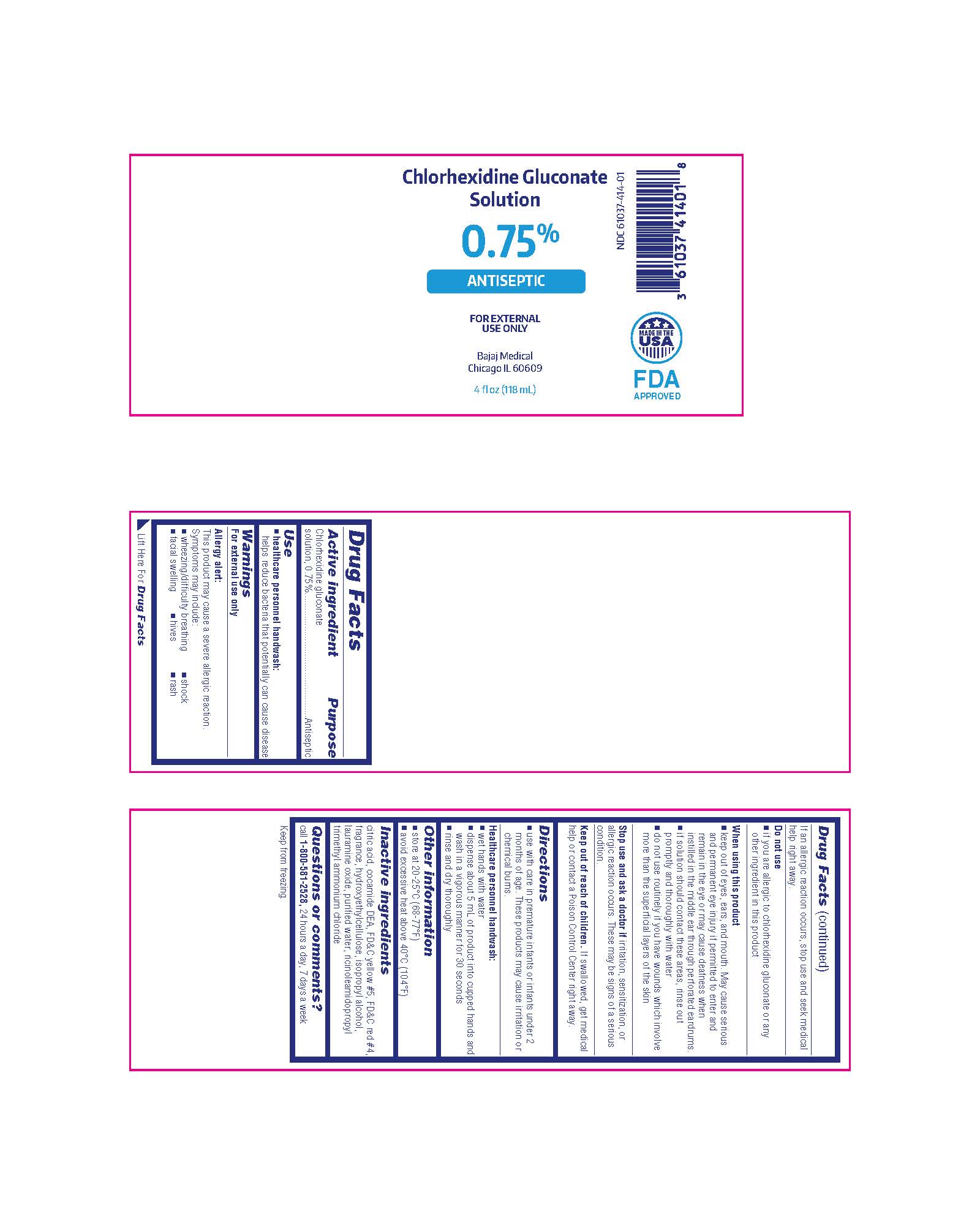
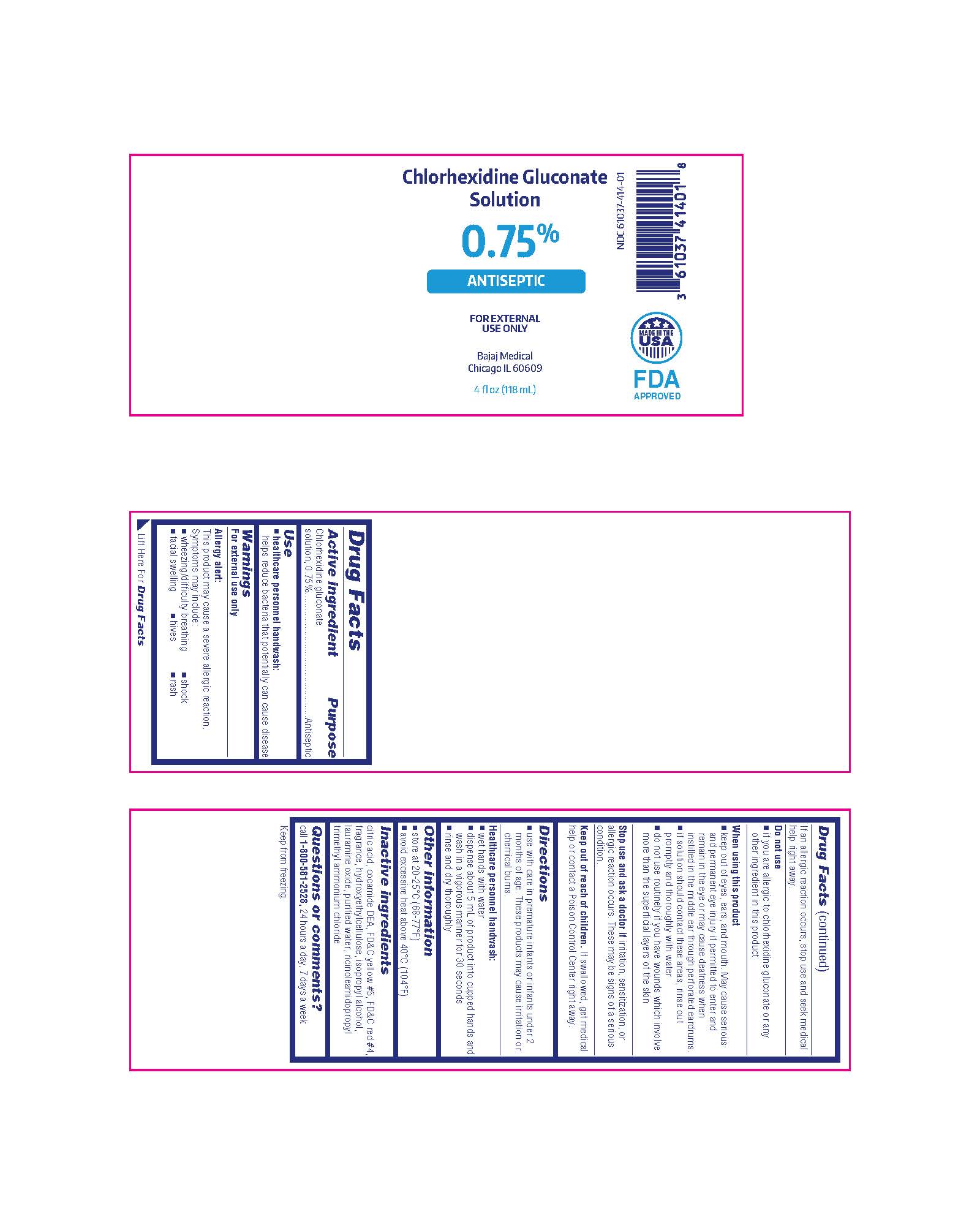
Package/Label Principal Display Panel
Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
4 fl oz (118 mL)
NDC 61037-414-01
MADE IN THE USA
FDA APPROVED
Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
8 fl oz (236 mL)
NDC 61037-414-02
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
16 fl oz (473 mL)
NDC 61037-414-05
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
32 fl oz (946 mL)
NDC 61037-414-06
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
128 fl oz (3785 mL)
NDC 61037-414-07
MADE IN THE USA
FDA APPROVED
Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
8 fl oz (236 mL)
NDC 61037-414-03
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
8 fl oz (236 mL)
NDC 61037-414-04
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
40.6 fl oz (1.2 L)
NDC 61037-414-08
MADE IN THE USA
FDA APPROVED

-
INGREDIENTS AND APPEARANCE
CHLORHEXIDINE GLUCONATE SOLUTION 0.75% ANTISEPTIC
chlorhexidine gluconate solution 0.75% antiseptic solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61037-414 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 0.75 mg in 100 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) HYDROXYETHYL CELLULOSE (140 CPS AT 5%) (UNII: 8136Y38GY5) ISOPROPYL ALCOHOL (UNII: ND2M416302) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) RICINOLEAMIDOPROPYLTRIMONIUM CHLORIDE (UNII: 93OU7D1C3U) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61037-414-01 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/30/2014 2 NDC:61037-414-02 236 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/30/2014 3 NDC:61037-414-05 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/30/2014 4 NDC:61037-414-06 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/30/2014 5 NDC:61037-414-07 3785 mL in 1 JUG; Type 0: Not a Combination Product 09/30/2014 6 NDC:61037-414-03 236 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/12/2017 7 NDC:61037-414-04 236 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/12/2017 8 NDC:61037-414-08 1200 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 10/12/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020111 09/30/2014 Labeler - Bajaj Medical, LLC (078774921) Registrant - Bajaj Medical, LLC (078774921) Establishment Name Address ID/FEI Business Operations Bajaj Medical, LLC 078774921 label(61037-414) , analysis(61037-414) , manufacture(61037-414) , pack(61037-414)