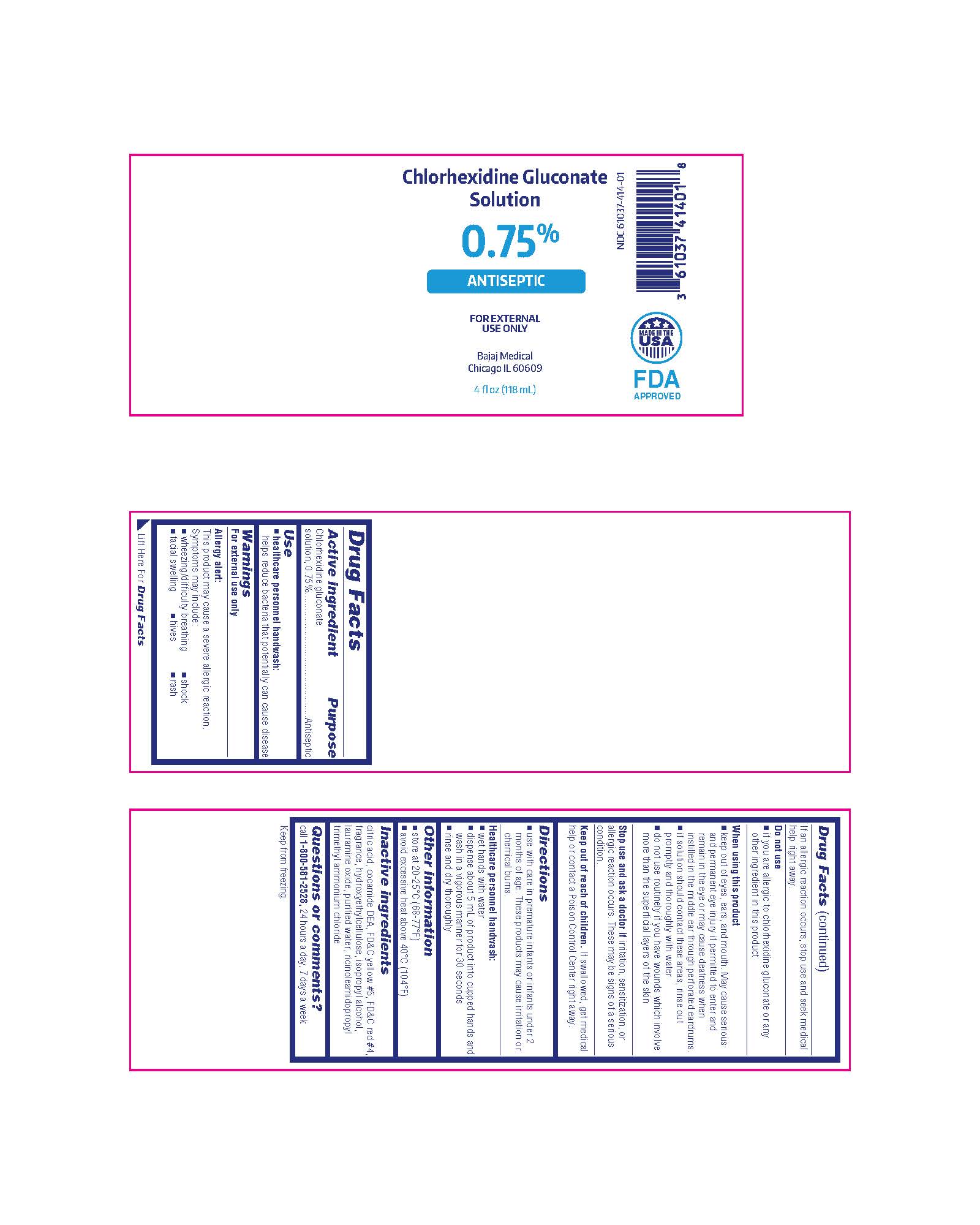
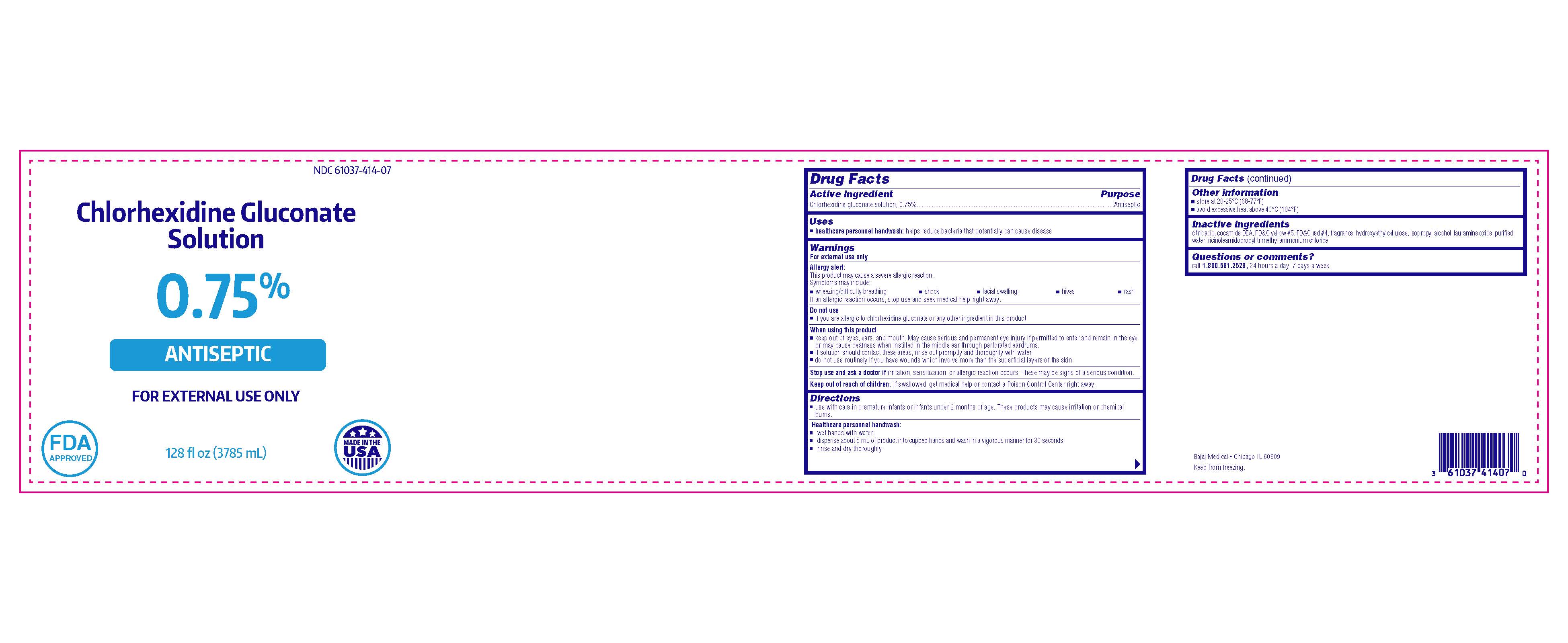
Warnings
For external use only
Allergy alert:
This product may cause a severe allergic reaction.
Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
Directions
- use with care in premature infants and infants under 2 months of age. These products may cause irritation or chemical burns.
Inactive ingredients
citric acid, cocamide DEA, FD&C yellow #5, FD&C red #4, fragrance, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, purified water, ricinoleamidopropyl trimethyl ammonium chloride
Package/Label Principal Display Panel
Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
4 fl oz (118 mL)
NDC 61037-414-01
MADE IN THE USA
FDA APPROVED
Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
8 fl oz (236 mL)
NDC 61037-414-02
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
16 fl oz (473 mL)
NDC 61037-414-05
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
32 fl oz (946 mL)
NDC 61037-414-06
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
128 fl oz (3785 mL)
NDC 61037-414-07
MADE IN THE USA
FDA APPROVED
Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
8 fl oz (236 mL)
NDC 61037-414-03
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
8 fl oz (236 mL)
NDC 61037-414-04
MADE IN THE USA
FDA APPROVED

Chlorhexidine Gluconate Solution
0.75%
ANTISEPTIC
FOR EXTERNAL USE ONLY
Bajaj Medical
Chicago IL 60609
40.6 fl oz (1.2 L)
NDC 61037-414-08
MADE IN THE USA
FDA APPROVED
