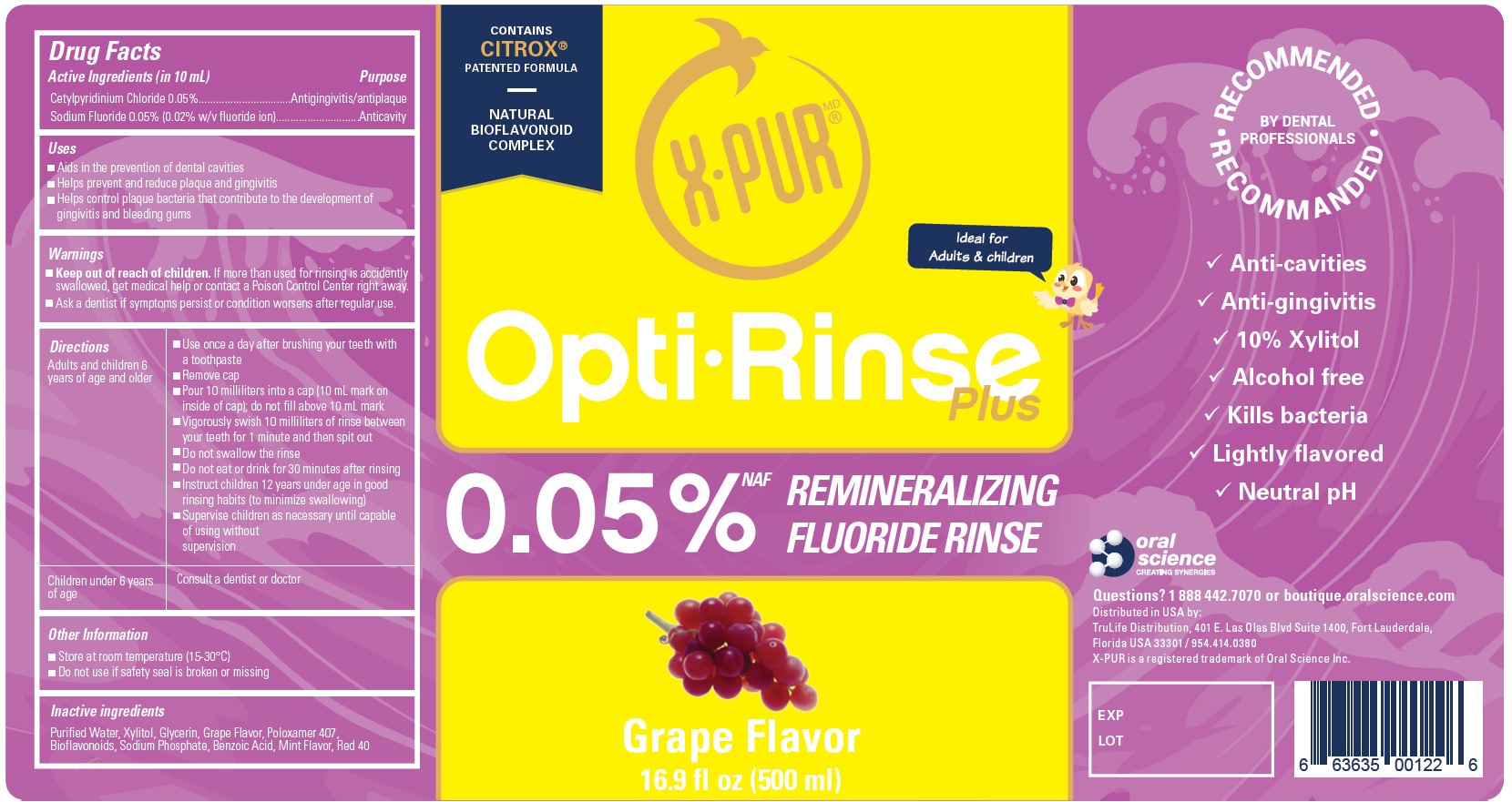
Label: OPTIRINSE PLUS- remineralizing fluoride rinse liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 77982-001-01 - Packager: Laboratoires MSP Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in 10 mL)
- Purpose
- Uses
- Warnings
- WARNINGS
-
Directions
Adults and children 6 years of age and older - Use once a day after brushing your teeth with a toothpaste
- Remove cap
- Pour 10 milliliters into a cap (10 mL mark on inside of cap); do not fill above 10 mL mark
- Vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out
- Do not swallow the rinse
- Do not eat or drink for 30 minutes after rinsing
- Instruct children 12 years under age in good rinsing habits (to minimize swallowing)
- Supervise children as necessary until capable of using without supervision
Children under 6 years of age Consult a dentist or doctor - Other Information
- Inactive Ingredients
-
OptiRinse Plus - Grape - 500 mL - NDC 77982-001-01
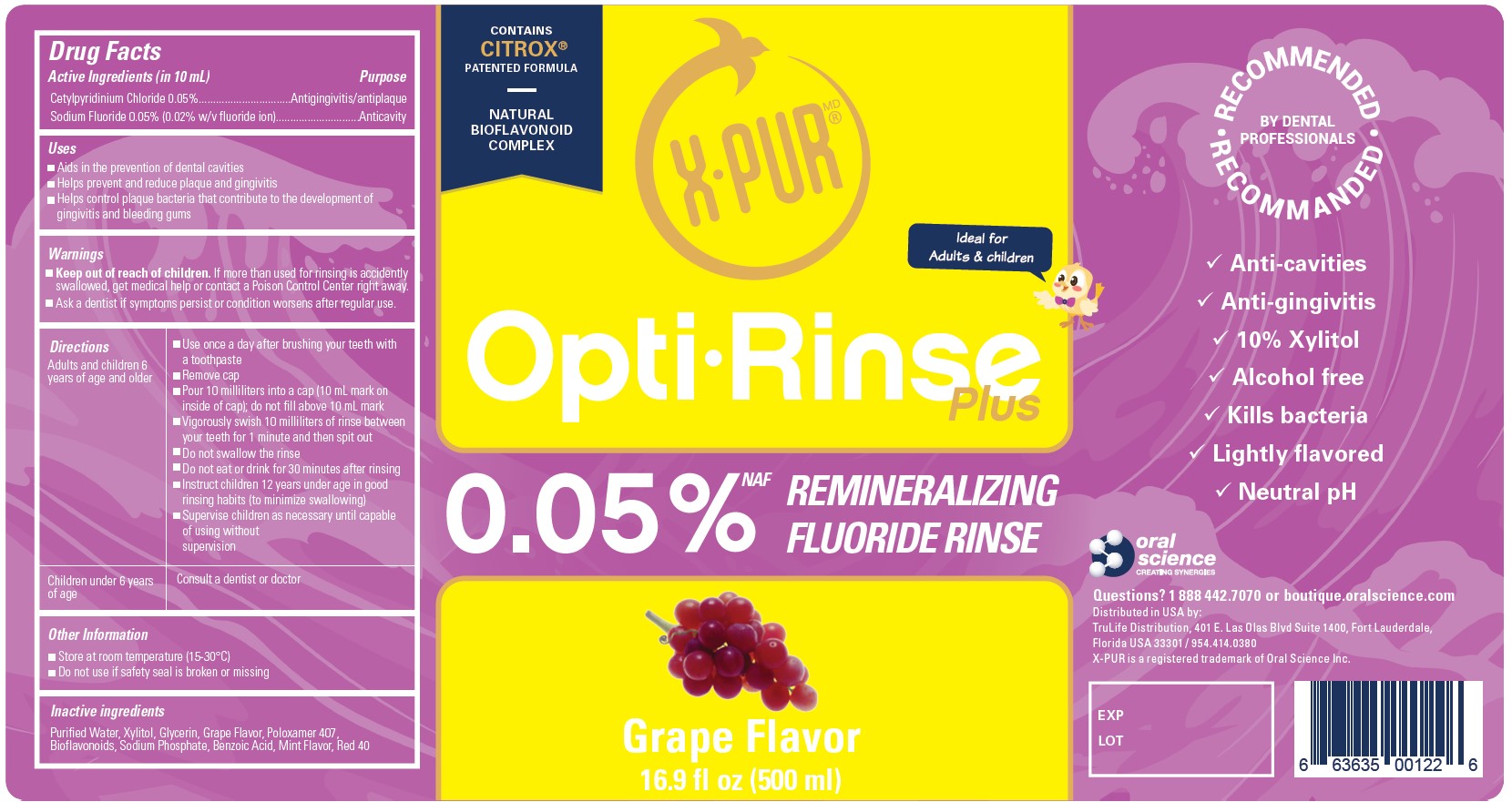
OptiRinse Plus
Contains Citrox patented formula
Natural Bioflavinoid Complex
Ideal for Adults & children
00.05% NAF Remineralizing Fluoride Rinse
Grape Flavor
16.9 fl oz (500 ml)
Recommended by Dental Professionals
- Anti-cavities
- Anti-gingivitis
- 10% Xylitol
- Alcohol free
- Kills bacteria
- Lightly flavored
- Neutral pH
Questions? 1 888 442.7070 or boutique.oralscience.com
Distributed in USA by:
TruLife Distribution, 401 E Las Olas Blvd Suite 1400, Fort Lauderdale,
Floirida USA 33301 / 954.414.0380
X-PUR is a registered trademark of Oral Science Inc.
-
INGREDIENTS AND APPEARANCE
OPTIRINSE PLUS
remineralizing fluoride rinse liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77982-001 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.05 mg in 1 mL SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.05 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) GLYCERIN (UNII: PDC6A3C0OX) METHYL ANTHRANILATE (UNII: 981I0C1E5W) POLOXAMER 407 (UNII: TUF2IVW3M2) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) SODIUM PHOSPHATE (UNII: SE337SVY37) BENZOIC ACID (UNII: 8SKN0B0MIM) PULEGONE (UNII: 4LF2673R3G) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color Score Shape Size Flavor grape Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77982-001-01 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 11/04/2020 Labeler - Laboratoires MSP Inc (251046124) Establishment Name Address ID/FEI Business Operations Laboratoires MSP Inc 251046124 manufacture(77982-001)