Active Ingredients (in 10 mL)
Cetylpyridinum Chloride 0.05%
Sodium Fluoride 0.05% (0.02% w/v fluoride ion)
Uses
- Aids in the prevention of dental cavities
- Helps prevent and reduce plaque and gingivitis
- Helps control plaque bacteria that contribute to the development of gingivitis and bleeding gums
Warnings
- Keep out of reach of children. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
| Adults and children 6 years of age and older |
|
| Children under 6 years of age | Consult a dentist or doctor |
Other Information
- Store at room temperature (15-30°C)
- Do not use if safety seal is broken or missing
Inactive Ingredients
Purified Water, Xylitol, Glycerin, Grape Flavor, Poloxamer 407, Bioflavonoids, Sodium Phosphate, Benzoic Acid, Mint Flavor, Red 40
OptiRinse Plus - Grape - 500 mL - NDC 77982-001-01
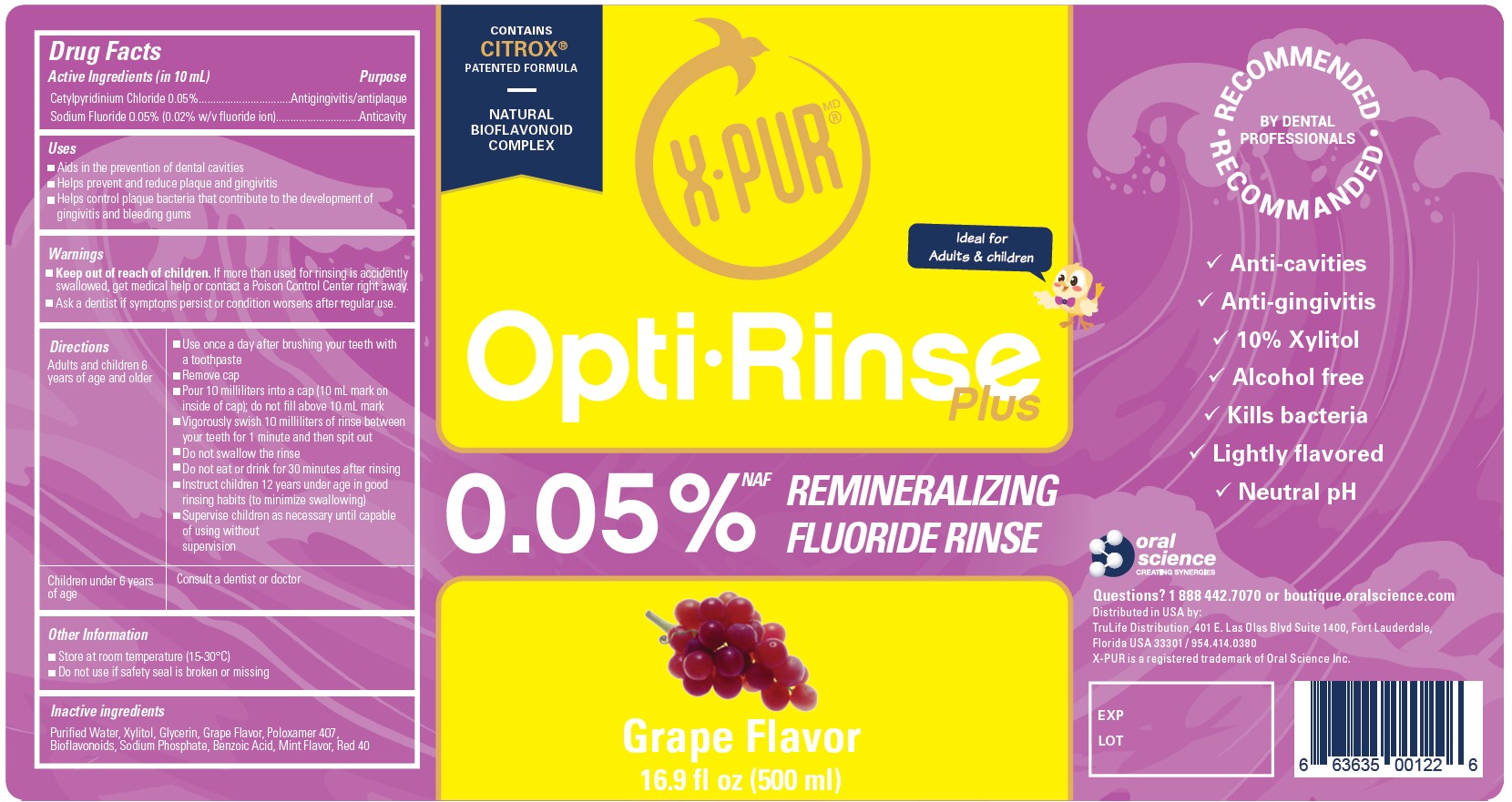
OptiRinse Plus
Contains Citrox patented formula
Natural Bioflavinoid Complex
Ideal for Adults & children
00.05% NAF Remineralizing Fluoride Rinse
Grape Flavor
16.9 fl oz (500 ml)
Recommended by Dental Professionals
- Anti-cavities
- Anti-gingivitis
- 10% Xylitol
- Alcohol free
- Kills bacteria
- Lightly flavored
- Neutral pH
Questions? 1 888 442.7070 or boutique.oralscience.com
Distributed in USA by:
TruLife Distribution, 401 E Las Olas Blvd Suite 1400, Fort Lauderdale,
Floirida USA 33301 / 954.414.0380
X-PUR is a registered trademark of Oral Science Inc.