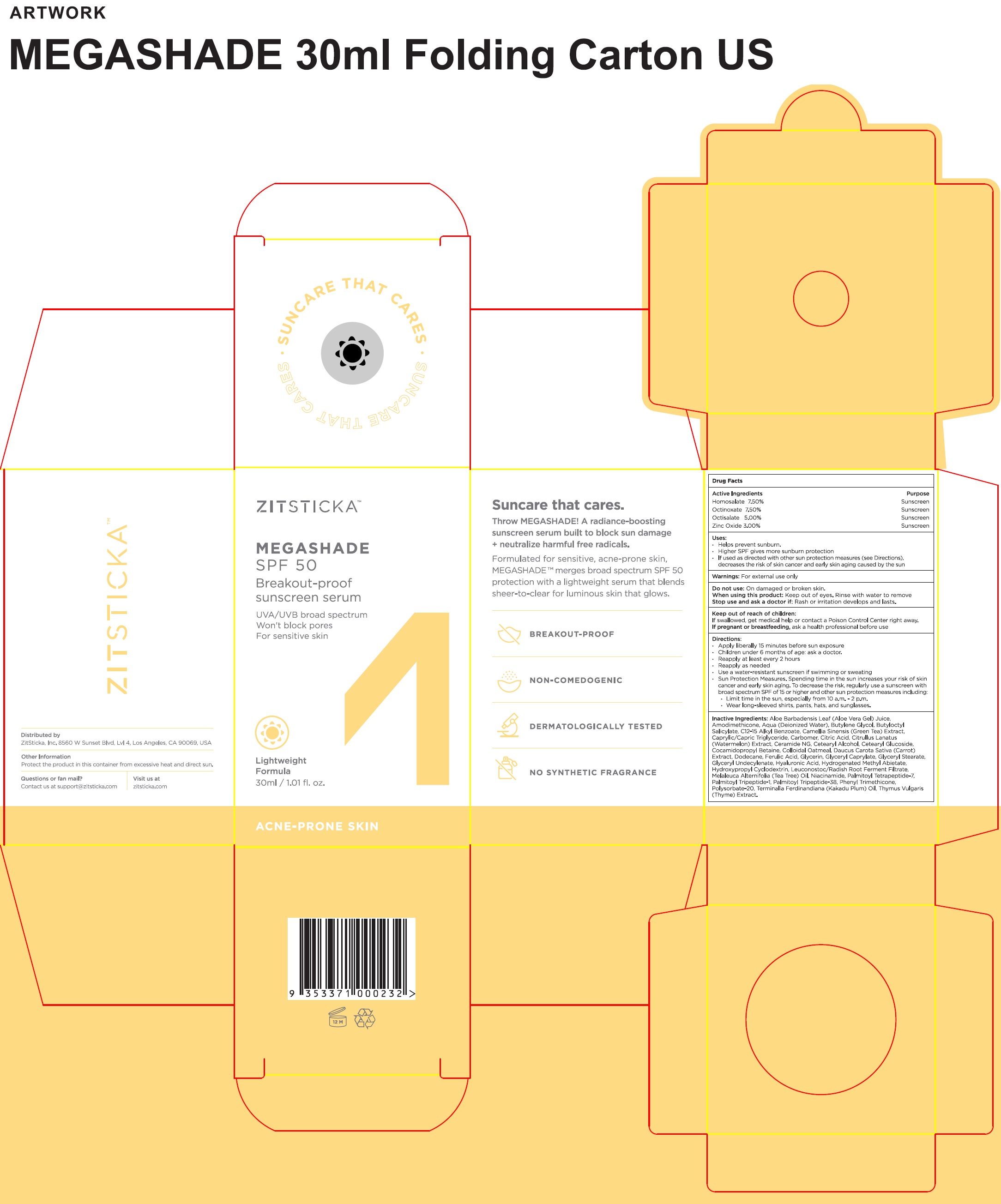
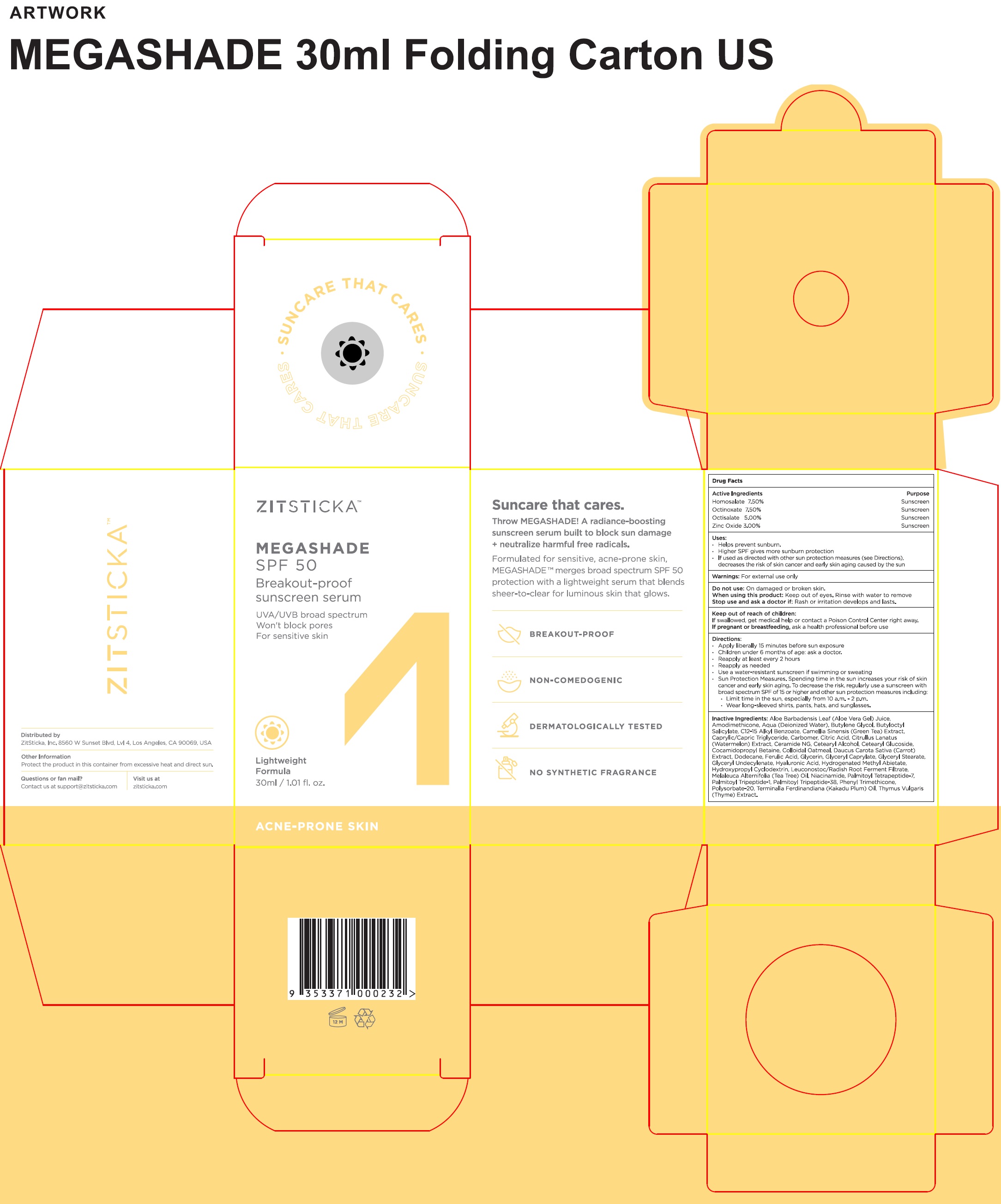
Label: ZITSTICKA MEGASHADE SUNSCREEN SERUM (SPF-50)- homosalate, octinoxate, octisalate, zinc oxide liquid
- NDC Code(s): 81746-183-00, 81746-183-01
- Packager: ZITSTICKA, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts.
- Active Ingredients
- Uses:
- Warnings:
-
Directions:
• Apply liberally 15 minutes before sun exposure • Children under 6 months of age: ask a doctor. • Reapply at least every 2 hours • Reapply as needed • Use a water-resistant sunscreen if swimming or sweating • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m. • Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Butylene Glycol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Camellia Sinensis (Green Teat) Extract, Caprylic/Capric Triglyceride, Carbomer, Citric Acid, Citrullus Lantus (Watermelon) Extract, Ceramide NG, Cetearyl Alcohol, Cetearyl Glucoside, Cocamidopropyl Betaine, Colloidal Oatmeal, Daucus Carota Sativa (Carrot) Extract, Dodecane, Ferulic Acid, Glycerin, Glyceryl Caprylate, Glyceryl Stearate Citrate, Glyceryl Undecylenate, Hyaluronic Acid, Hydrogenated Methyl Abietate, Hydropropyl Cyclodextrin, Leuconostoc/Radish Root Ferment Filtrate, Melaleuca Alternifolia (Tea Tree) Oil, Niacinamide, Palmitoyl Tetrapeptide-7, Palmitoyl Tripeptide-1, Palmitoyl Tripeptide-38, Phenyl Trimethicone, Polysorbate-20, Terminalia Ferdinandiana (Kakadu Plum) Oil, Thymus Vulgaris (Thyme) Extract.
- Other Information:
- Questions?
- Package Labeling:81746-183-00
- Package Labeling:81746-183-01
-
INGREDIENTS AND APPEARANCE
ZITSTICKA MEGASHADE SUNSCREEN SERUM (SPF-50)
homosalate, octinoxate, octisalate, zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81746-183 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 75 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATERMELON (UNII: 231473QB6R) CERAMIDE NG (UNII: C04977SRJ5) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) OATMEAL (UNII: 8PI54V663Y) CARROT (UNII: L56Z1JK48B) DODECANE (UNII: 11A386X1QH) FERULIC ACID (UNII: AVM951ZWST) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) HYALURONIC ACID (UNII: S270N0TRQY) HYDROGENATED METHYL ABIETATE (UNII: A23O709X8O) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) NIACINAMIDE (UNII: 25X51I8RD4) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PALMITOYL LYSYLDIOXYMETHIONYLLYSINE (UNII: T7A529FB8O) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYSORBATE 20 (UNII: 7T1F30V5YH) KAKADU PLUM (UNII: 0ZQ1D2FDLI) THYME (UNII: CW657OBU4N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81746-183-00 1 in 1 BOX 04/10/2021 1 50 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:81746-183-01 1 in 1 BOX 09/01/2021 2 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/10/2021 Labeler - ZITSTICKA, Inc (117778611)