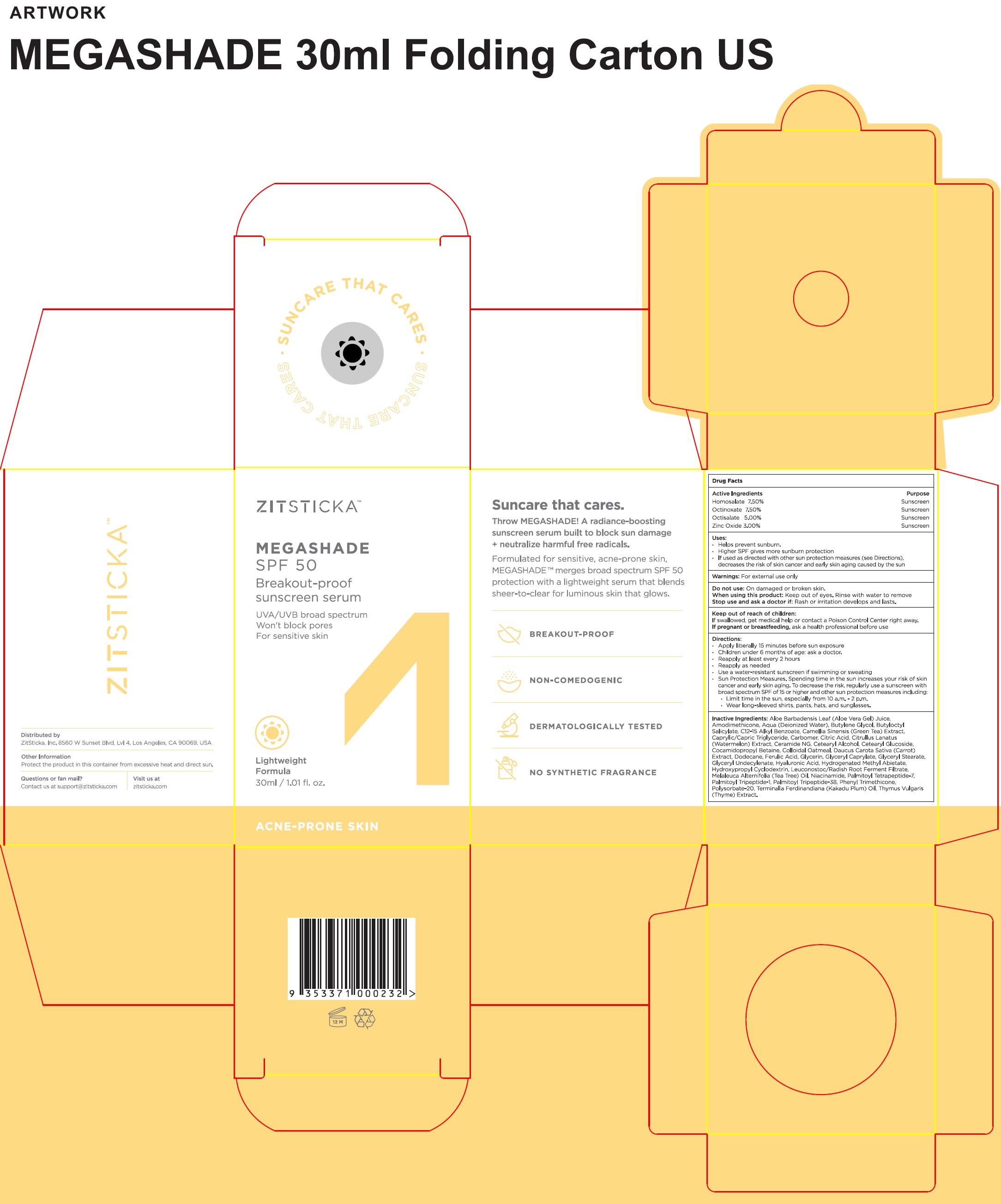
Uses:
- Helps prevent sunburn.
- Higher SPF gives more sunburn protection
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings:
For external use only
Directions:
• Apply liberally 15 minutes before sun exposure • Children under 6 months of age: ask a doctor. • Reapply at least every 2 hours • Reapply as needed • Use a water-resistant sunscreen if swimming or sweating • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m. • Wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Butylene Glycol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Camellia Sinensis (Green Teat) Extract, Caprylic/Capric Triglyceride, Carbomer, Citric Acid, Citrullus Lantus (Watermelon) Extract, Ceramide NG, Cetearyl Alcohol, Cetearyl Glucoside, Cocamidopropyl Betaine, Colloidal Oatmeal, Daucus Carota Sativa (Carrot) Extract, Dodecane, Ferulic Acid, Glycerin, Glyceryl Caprylate, Glyceryl Stearate Citrate, Glyceryl Undecylenate, Hyaluronic Acid, Hydrogenated Methyl Abietate, Hydropropyl Cyclodextrin, Leuconostoc/Radish Root Ferment Filtrate, Melaleuca Alternifolia (Tea Tree) Oil, Niacinamide, Palmitoyl Tetrapeptide-7, Palmitoyl Tripeptide-1, Palmitoyl Tripeptide-38, Phenyl Trimethicone, Polysorbate-20, Terminalia Ferdinandiana (Kakadu Plum) Oil, Thymus Vulgaris (Thyme) Extract.