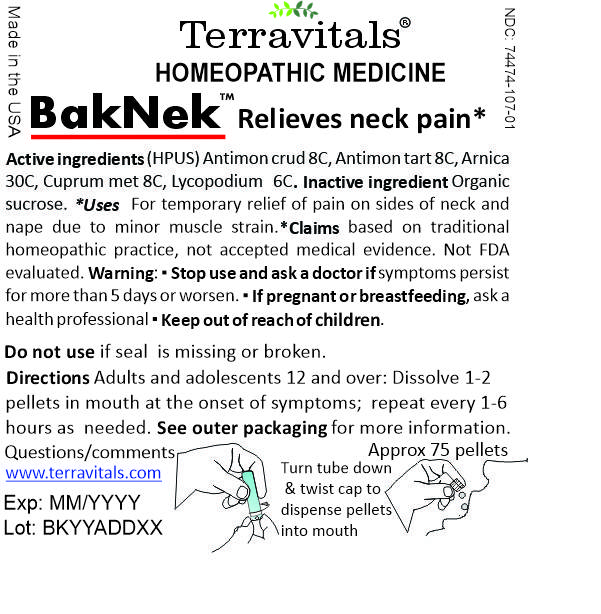
Label: TERRAVITALS BAKNEK- antimonium crudum, antimonium tartaricum, arnica montana, cuprum metallicum, lycopodium clavatum pellet
- NDC Code(s): 74474-107-01
- Packager: Terravitals LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 1, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Terravitals BakNek Active Ingredients
Drug Facts
Active ingredients
Antimonium crudum 8C HPUS
Antimonium tartaricum 8C HPUS
Arnica montana 30C HPUS
Cuprum metallicum 8C HPUS
Lycopodium clavatum 6C HPUS
'HPUS' indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States.
Small inner packaging label:
-
Terravitals BakNek Purpose
Active ingredients** ...................................................................... Purpose
Antimonium crudum 8C HPUS...............neck/back pain, neck/nape cramp
Antimonium tartaricum 8C HPUS .............. stiffness aggravated by motion
Arnica montana 30C HPUS................weakness and painful glands of neck
Cuprum metallicum 8C HPUS.. swelling neck glands,muscles back to neck
Lycopodium clavatum 6C HPUS.............painful stiffness of left side of neck
'HPUS' indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States
**'C' is homeopathic dilution.
Uses* For temporary relief of pain on sides of neck and nape due to minor muscle strain.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- Terravitals BakNek Warnings
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- Terravitals BakNek Dosage
- Terravitals BakNek Other
- DO NOT USE
- Terravitals BakNek Inactive Ingredient
-
Terravitals BakNek main panel
TERRAVITALS BakNek
Homeopathic Medicine
Relieves neck pain*
NDC 74474-107-01
Approx 75 Oral soluble pellets
Non-drowsy
Not habit-forming
No drug interactions
No known side effects
Made in the USA by Terravitals LLC, Gaithersburg MD 20879
www.terravitals.com
Exp: MM/YYYY
Lot: BKYYADDXX
Outer packaging label:

-
INGREDIENTS AND APPEARANCE
TERRAVITALS BAKNEK
antimonium crudum, antimonium tartaricum, arnica montana, cuprum metallicum, lycopodium clavatum pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74474-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 8 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_C] ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 8 [hp_C] ARNICA MONTANA WHOLE (UNII: O80TY208ZW) (ARNICA MONTANA WHOLE - UNII:O80TY208ZW) ARNICA MONTANA WHOLE 30 [hp_C] COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 8 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Product Characteristics Color white (White spheroids) Score Shape Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74474-107-01 1 in 1 TUBE 04/19/2021 1 1 in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/19/2021 Labeler - Terravitals LLC (117322434) Establishment Name Address ID/FEI Business Operations Terravitals LLC 117322434 manufacture(74474-107)

