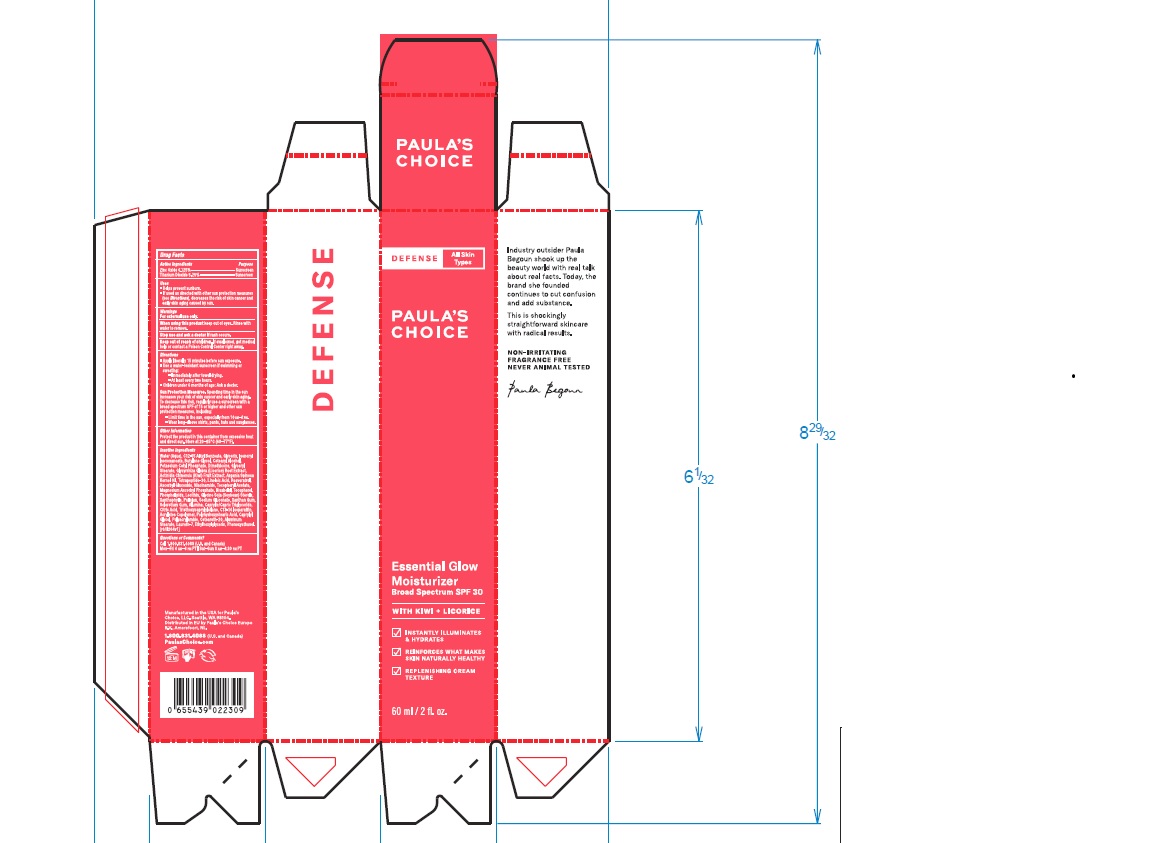
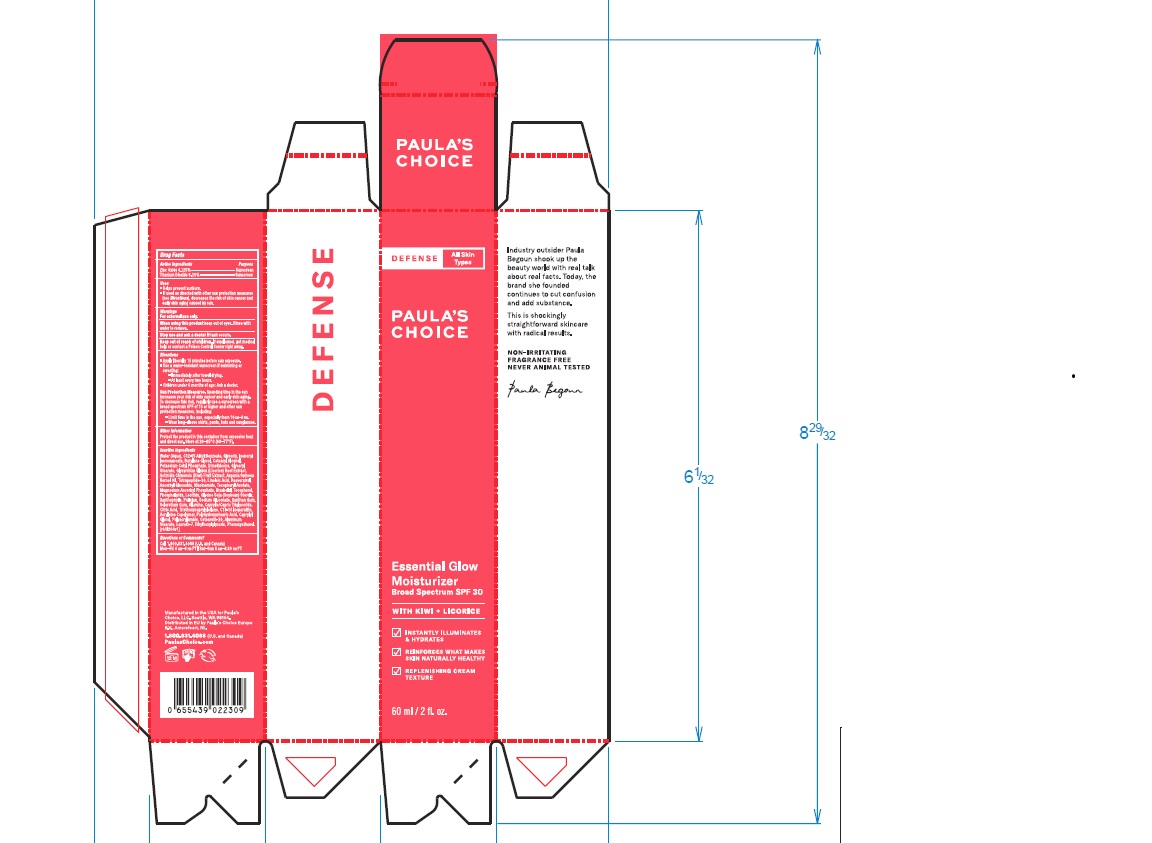
Label: PAULAS CHOICE DEFENSE ESSENTIAL GLOW MOISTURIZER SPF 30- zinc oxide, titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 76144-223-01, 76144-223-02 - Packager: Paula's Choice LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INSTRUCTIONS FOR USE
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Water (Aqua), C12-15 Alkyl Benzoate, Glycerin, Isononyl Isononanoate, Butylene Glycol, Cetearyl Alcohol, Potassium Cetyl Phosphate, Dimethicone, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Root Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Argania Spinosa Kernel Oil, Tetrapeptide-30, Linoleic Acid, Resveratrol, Ascorbyl Glucoside, Niacinamide, Tocopheryl Acetate, Magnesium Ascorbyl Phosphate, Bisabolol, Tocopherol, Phospholipids, Lecithin, Glycine Soja (Soybean) Sterols, Xanthophylls, Pullulan, Sodium Gluconate, Xanthan Gum, Sclerotium Gum, Alumina, Caprylic/Capric Triglyceride, Citric Acid, Triethoxycaprylylsilane, C13-14 Isoparaffin, Acrylates Copolymer, Polyhydroxystearic Acid, Caprylyl Glycol, Polyacrylamide, Ceteareth-20, Aluminum Stearate, Laureth-7, Ethylhexylglycerin, Phenoxyethanol.
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAULAS CHOICE DEFENSE ESSENTIAL GLOW MOISTURIZER SPF 30
zinc oxide, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76144-223 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 61.25 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 52.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) LEVOMENOL (UNII: 24WE03BX2T) LUTEIN (UNII: X72A60C9MT) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) SODIUM GLUCONATE (UNII: R6Q3791S76) XANTHAN GUM (UNII: TTV12P4NEE) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) ALUMINUM STEARATE (UNII: U6XF9NP8HM) LAURETH-7 (UNII: Z95S6G8201) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOY STEROL (UNII: PL360EPO9J) ETHYL ACRYLATE/METHACRYLIC ACID/STEARETH-20 METHACRYLATE COPOLYMER (UNII: EPA1872R1N) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PULLULAN (UNII: 8ZQ0AYU1TT) BETASIZOFIRAN (UNII: 2X51AD1X3T) ALUMINUM OXIDE (UNII: LMI26O6933) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POLYACRYLAMIDE (1500 MW) (UNII: 5D6TC4BRWV) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) KIWI FRUIT (UNII: 71ES77LGJC) ARGAN OIL (UNII: 4V59G5UW9X) LINOLEIC ACID (UNII: 9KJL21T0QJ) RESVERATROL (UNII: Q369O8926L) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) NIACINAMIDE (UNII: 25X51I8RD4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76144-223-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 09/10/2018 2 NDC:76144-223-01 60 mL in 1 TUBE; Type 0: Not a Combination Product 09/10/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/10/2018 Labeler - Paula's Choice LLC (029583981)